NOMENCLATURE,
DESCRIPTION, AND DISCUSSION
1. Taxus wallichiana
Zuccarini, in Siebold & Zuccarini, Abh. math.-phys Cl. k. Bayer.
Akad. Wiss. (München) 1 (3): 803, Tab. 5 (Fig. 9). 1843. Taxus
baccata subsp. wallichiana (Zucc.) Pilger in Engler,
Pflanzenreich IV (5): 112. 1903. Taxus baccata var. wallichiana
C. K. Schneider ex Silva Tarouca, Freiland-Nadelgehölz. 276. 1913. No
specimens cited by Zuccarini, original material at M: specimens from NEPAL,
INDIA. Lectotype (designated by Spjut in adnot. 23 Mar 1995, in
J. Bot. Res. Inst. Texas 1: 230. 2007): specimen with male cones, “Herb.
Zuccarini”—India: eastern, communicavit Wallich, year 1835—Wallich
s.n. (Fig. 10, M!). Other related material: Wallich
6054A, p.p.,“Kumaon,” with Schultes label, in adnot. Torreya
nucifera, Taxus nucifera, and Taxus wallichiana, one large
branch with mature male cones (M!). Duplicates of Wallich 6054 (see below): GH!
K! NY! P! PH! S!
1a. Var. wallichiana. Shrub or tree to 20 m or more;
branchlets yellowish green, or reddish orange, becoming gradually darker
in age, or abruptly dark purplish (maroon) in 2nd yr;
bud-scales persistent at base of 1–2 yr branchlets, these pale
yellowish to brownish red (maroon), usually numbering 5–10,
overlapping in 3–4 ranks, the lower scales loosely adnate, ovate, ca.
0.5 mm long, upper scales spreading, concave and incurved towards apex
(cuspidate), to 1 mm long. Leaves ± evenly spreading but not evenly
distributed, linear, acuminate, straight to commonly falcate, 1.5–3.5
cm long, 1.5–2.5 mm wide, usually ca. 350 µm thick, thinner in plants
of NE India bordering Myanmar and Tibet, glossy (resinous) dark green
above, paler below, convex on upper surface to a rounded midrib, less
concave below to a flush to slightly rounded midrib, becoming revolute
near margins (80–90º), especially in upper third of dried leaves;
upper (adaxial) epidermal cells angular in x-sect., rectangular,
quadrangular, or taller than wide, commonly ca. 25 µm diam., or 20–30
µm tall and 20–40 µm wide; abaxial epidermis papillose except for
(2-) 4 (-6) cells across from margins, or 4–6 (-8) cells wide
in plants from Mt. Emei (Sichuan), the epidermal cells usually not
inflated, short rectangular, 1.5–3 times (×) longer than wide (l/w),
gradually becoming quadrate or short trapezoidal but not
particularly narrower towards margin, occasionally long rectangular near
margins in plants from Nepal (4–8× l/w) but then not tall, usually
8–12 µm tall, (10-) 15–25 µm wide, similar in shape and length on
midrib, or often narrower and longer on midrib, 3–10× l/w; papillae
usually distinct, often aggregate, positioned more marginally than
medially in 2–3 alternate rows, or medial in some specimens but then
cells not inflated; stomata usually 11–18 (-21) rows in yellowish
green to reddish orange bands (dried leaves), the stomata rows generally
decreasing in number from east to west, palisade parenchyma 1 row,
generally 50–70 µm long; spongy parenchyma cells ellipsoidal to
bone-like, forming a periclinal net, not falling apart when sectioned.
Male cones maturing on 1st and 2nd yr branchlets,
ca. 1.5 wide and 4 mm long in bud, to 2 mm wide and 6 mm long at
maturity. Female cones maturing on 1st or 2nd yr
or older branchlets; seed conical in upper third, 6 mm long, 4 mm diam.,
slightly thickened at base, recessed at attachment point, with red or
yellow aril.
Wallich yew. Distribution: E Himalayas to SW China; montane
coniferous forests with Picea, Abies, Tsuga, or broadleaved
evergreen forests of Lithocarpus, or Quercus, (1500-)
2300–3200 m; C & E Nepal, Bhutan, NE India (Assam, Manipur, Khasia Hills,
West Bengal), Myanmar, China (SE Tibet, Sichuan, Yunnan). In Nepal evidently
occurring abundantly with Abies spectabilis (D. Don) Spach on
limestone (Stainton 1972), and in Bhutan apparently scattered from Ha to
Mongar districts (Grierson and Long 1983).
Representative
Specimens—Nepal:
Arun Valley, N of Kutiar, 9000 ft., Stainton et al. 1398 (BM);
Eastern Nepal, Duon Kosi, Chaunrikarua, 27º40'N, 86º40'E, 9500
ft, Stainton et al. 6601 (BM); Stainton et al. 4496 (BM); Solukhumbu Dist., Dudh Kosi River,
Lamujo to Chumava, 2450 m, Hideo Tabata et al. 10585 (A, BM);
Thulo Kobar to Ran Thanti, 83º45’ E, 28º24’N, 2600 m, Ohba et
al. 8310264 (BM). Bhutan: Tunle La, near Kinga Rapden, 27º27’N,
90º37’E, 11,000 ft, Ludlow et al. 18672 (BM, GH); Thimphu
Dist., summit of Dochong La, 27º29’ 89º45, Tsuga/Rhododendron
forest, 3110 m, Grierson & Long 4417 (A); 7500 ft, Cooper
& Bulley 2600 (BM); above Motithang directly W of Thimphu, Bartholomew
& Boufford 3917 (A); illegible, 9000-10,000 ft, Griffith
2006 (BM, K, p.p., with T. sumatrana). India—West Bengal:
Singalila Range, along trail from Rimbick to Sandakphu, 8400 ft, very
large tree with circumference near base of 11’6", Voss et al.
148 (NA). Assam: without additional locality data, Griffith
2706 (BM). Khasia [Meghalaya]: 4500 ft, Vale of rocks, C.
B. Clarke 436743 (BM); Khasia, 5000–6000 ft, J. D. Hooker 77,
87 (GH, p.p., lower left of 3 specimens on one sheet; P; PH).
East Himalaya, without locality, Griffith 5002 (P: 2
sheets, one distributed by K, the other ex Herb. Bunge). Manipur:
Seriphari, 10,000 ft, Jan. 1882, G. Watt 5955 (GH, P), 6208
(P), 6493 (P). Sirhoi: 8000 ft, small tree, scattered in forest,
not common, only conifer above 7000 ft, Kingdon Ward 17271 (A,
BM). India (without specific locality data), K. Biswas 439 (A,
US). Myanmar (Burma): Northern: Adung Valley, 27–28º30'N,
97–98º30'E, 7000-8000 ft, shrub, of dry forest, Kingdon Ward 9375
(A); Adung Valley, 6000 ft, shrub, scattered through the thickets along
river, Kingdon Ward 9214 (A, BM). China—SE Tibet:
Cha Yu, 2370 m, tree ca. 15 yrs in age, occurring with Pinus sp.,
ChaYu Forestry Bureau Staff Expert CYW007 (ChaYu Forestry Bureau). Sichuan:
Mt. Emei [Shan], 2300 m, Yu-shih Lin 1196 (A, US); 2400 m, T.T.
Yu 482 (A), 492 (A); W. P. Fang 3945 (A); W.K. Hu
8166
(A); T.C. Lee
4465 (A); Pan-lan-shan W of Kuan Hsien, 5000–6000 ft, Wilson
4053 (A, BM, US). Yunnan: Salween, Kiukiang Divide, Shawlongwang,
2600 m, among mixed forest in valley, tree 40–60 ft, bark purplish
brown, thinly scaly, leaves dark green above, yellowish beneath, seed
brownish green, half covered by the coral red fleshy aril, rare, Nov
1938, T.T. Yu 21036. Cultivated—India: Dehra Dun,
Bot. Gard. Darjeeling, Raijada 18919 (A). Specimen data
questionable: Uttar Pradesh (Kumaon): Blinkworth s.n., in
adnot. T. virgata (BM), probably not collected by Blinkworth and
probably not collected in Kumaon.
Duplicates of Original Material (Isosyntypes)
by institution: GH: label with handwriting similar to
Wallich, “Taxus nucifera Wall.” “Napalia.” s.n. K:
Four sheets. (1) with two specimens, the larger specimen has a pasted
label below it with handwriting “6054a” and no indication of
locality data, the smaller one is a single branch with mature seed,
correctly annotated T. wallichiana by S. G. Harrison, but it is
not 6054A, or not a type since it is not from a male plant; (2)
two specimens, one large specimen with an imprinted stamp nearby—Herb.
Hookerianum, with handwriting similar to Wallich, 6054/A, Nepal,
accompanied by a smaller specimen in left corner, with a large label
below, Watt 6493 from Munipur, det. T. wallichiana by
Spjut; (3) has four specimens, but only the lower left specimen is a
type (T. wallichiana), below it are several labels, one
printed—ex Herb. George Gordon, presented by J. D. Hooker,
1878, the other bears handwritten annotation—"Taxus
wallichiana," two largest specimens with letters a and b
written nearby on left and right, respectively, and with Herb. Benthamum
imprinted in center, belong to T. contorta; uppermost annotated T.
virgata, det. by Spjut to be a young shoot of T. baccata. NY:
2 sheets, 6054A, NY accession numbers 30328 and 30329 (det. via
photocopy). P: “Napalia,” 6054 with “A” inserted,
annotated Taxus nucifera Kaempf.? on label ex. Herb. Richard, and
additional label ex. Herb. E. Drake. PH: “6054A Wallich.”
|
|
 
Lectotype (M): India, eastern, with annotation label
by R. Spjut, 23 Mar 1995. Specimen on right shows closer view
of male cones and persistent scales at base of branchlets.
|
 

India-Assam: Griffth 2(7)606 (BM). Right
photo shows close-up of male cones and persistent scales at base of
branchlets, which are rather pale yellowish-green to pale orange.
Sketch on packet indicates abaxial leaf margin has 4 rows of smooth
cells followed by 7 rows of papillose cells, 15 stomata rows and a
midrib 15 cells wide; papillae are marginal (alternate) on both
midrib and marginal cells. |
 

India-Khasia [Meghalaya]
J. D. Hooker 77 (GH), p.p. lower left of 3 specimens, T. wallichiana, shown in
closer view on right; right specimen in left photo, T. contorta;
upper specimen in left photo, T. sumatrana. Note loose
pale scales (but similar in color to branchlets) at base of
branchlets in right photo. Specimens from China that have
similar scales but shorter (oblong) leaves are referred to T.
scutata (see examples of T. scutata in
Chinensis Subgroup). Lower photo, specimen at PH.
Leaves from duplicates at P and at US were found to be identical in
having 4 smooth marginal cells followed by 7 rows of papillose
cells, 16 rows of stomata and 15 midrib cells with marginal
papillae. |
  India-Manipur:
Watt 5955 (P). A leaf from this and from an identical specimen in A were
found to have 17 stomata rows per band. |
|

Bhutan: Thimphu District, Grierson & Long 4417 (A).
Photos and packet attached to specimen, all returned to A thru
NA in July 1996. Upper photo shows stomata band, lower photo
shows midrib with marginal papillae (along cell walls), also
indicated in illustration on packet. |
 

Bhutan: W of Thimphu, Bartholomew
& Boufford 3917 (A). |
  Sichuan:
Mt. Emei, W. K. Hu 8166 (A). Photo on left shows
authors sketch (returned with loan). Abaxial surface of leaf
indicated to have 4(-6) rows of marginal cells followed by 9 rows of
papillose cells and 18 rows of stomata. Leaf x-section shows
reddish rectangular epidermal cells. Photo on right shows sharp
contrast between young pale orange and older reddish purple
branchlets with persistent scales at base of branchlets. |

Sichuan:
Mt. Emei, 2300 m, Yu-shih Lin 1196 (A). Illustration and
photo attached to specimen and further enlarged by separate scan.
Photo shows abaxial surface of leaf, stomata bands in part and midrib region
magnified 250x. Here stomata can be seen on the narrow midrib.
Papillae mostly alternate in position along epidermal cells
(marginal papillae).
|
 
Nepal:
10,000 ft., Stainton et al. 8296 (BM). Sketch of
x-section of leaf in mid region shows distinct quadrangular
epidermal cells; lower part of sketch indicates abaxial leaf margin
has 3 rows of smooth cells followed by 8 rows of papillose cells,
and a stomata band with 11 rows of stomata; papillae are shown to be
alternate along narrow midrib cells and on wider marginal cells.
Leaves of this plant are shaped similarly to T. contorta,
while the scales at the base of branchlets are more like T.
wallichiana. Number of stomata rows and geographical
occurrence are borderline between the two species. Leaf shape
in x-section, absence of reddish parenchyma cells in the
mesophyll region, and angled pointed seed are features that agree
more with T. wallichiana. |
  Nepal: Solukhumbu Dist., Dudh Kosi River,
Lamujo to Chumava, 2450 m, Hideo Tabata et al. 10585 (A).
Sketch of leaf sections shows distinct quadrangular in leaf
x-section, and abaxial margin to have 4 rows of smooth cells
followed by 6 rows of papillose cells and a stomata band with 12
stomata rows. A leaf from a specimens at BM was found to have 11
stomata rows, and the epidermal cells were papillose to near the margin.
|
  Myanmar (Burma). Northern: Adung Valley, 27–28º30'N,
97–98º30'E, 7000-8000 ft, shrub, of dry forest, Kingdon Ward 9375
(A). Packet shows sketch of leaf sections. A x-section indicates
epidermal cells are quadrangular, 25
µm wide & tall; the abaxial leaf margin is
indicated to lack papillae across 4 cells, followed by 10 rows of
papillose cells, and a stomata band has 19 rows of stomata;
papillae are noted to be marginal.
|
 
Yunnan: Salween, Kiukiang Divide, Shawlongwang, 2600 m, T.T.
Yu 21036 (A). Image on right reproduced from Kwei,
Y-l. and
S-y. Hu. 1974. Epidermal feature of leaves of Taxus
in relation to taxonomy]. Acta Phytotax. Sin. 12(3): 329-334, plate
67, Fig. 1, referred to as T. wallichiana var. chinensis.
Abaxial leaf surface x65, showing midrib and stomata bands on each
side. Leaf shape in cross-section compares with var.
wallichiana, but general leaf shape and arrangement along with
position of papillae agree more with var. yunnanensis. |
|
The lectotype was selected from several Wallich specimens among
Zuccarini collections at München that best matched the illustration
(Siebold & Zuccarini 1843, Tab. 5); however, Zuccarini (Siebold
& Zuccarini 1843) did not cite specimens. Thus, the Wallich
specimens of T. wallichiana studied by Zuccarini might appear to
be syntypes, while those in other
herbaria not seen by Zuccarini could be considered isosyntypes.
This would be the case according to the International Code of Zoological
Nomenclature; however, the ICBN is not clear on this issue. Thus,
uncited specimens have to be referred to as original material or
duplicates of original material.
Zuccarini was
one of many recipients of specimens distributed by Wallich who generally
assigned collection numbers to species rather than to specimens in which
a particular specimen number may come from different localities.
For example, Wallich 6054A has been reported from Central
Midlands near Kathmandu Valley in Nepal fide Hara et al. (1978)
and Anonymous (1913), or “Cachemiro” fide Parlatore (1868),
whereas the lectotype—without number—was reportedly from eastern
India. The lectotype was indicated
by Zuccarini to have been collected or sent to him in the year 1835,
after distribution of Wallich 6054, sometime
between
1831 and 1832 (Anonymous 1913). It was probably collected in
Assam where Wallich was know to have collected during 1835 (Burkhill
1965). Because the ICBN (Art. 8.2) links the type to a single gathering,
isolectotypes cannot be recognized in this case. It should be noted that
types have male cones, reddish orange branchlets, non-inflated epidermal
cells on abaxial surface of leaves, and 12-15 rows of stomata/band.
Wallich specimens of
T. wallichiana with notations of “Kumaon” may be an error in
numbering or labeling since collections from “Kumaon” generally
belong to T. contorta, whereas Wallich collections without number
may indicate uncertainty of taxonomic assignment.
|
|
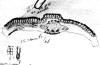
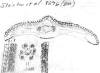
Fig.
9–10: Taxus wallichiana Zucc. Fig. 9 (left): Illustration by
Zuccarini in Siebold &
Zuccarini (1843). Fig. 10 (right): lectotype, Wallich s.n.,
reportedly from E India (M).
|
| Typical T. wallichiana is
identified by its pale reddish orange branchlets, its persistent
cuspidate bud-scales (Pilger 1916), its linear leaves arcuate
near base (Orr 1937; Pilger 1903), its conically shaped seeds (Orr 1937)
that often mature on 2nd yr or older branches (in the
Himalayas), and its angularly shaped epidermal leaf cells in x-section.
Its leaves are further distinguished from those of T. contorta by
the adhesive parenchyma cells, and by (11-) 13–18 (-21) rows of
stomata/band. Plants from Nepal, West Bengal, Khasia, and Bhutan compare
favorably with the type. Those from Myanmar, China, Vietnam, and Malesia
generally differ. This variation is discussed below in regard to
taxonomic and nomenclatural problems.
Taxus wallichiana has been the name applied to all yews in
southeastern Asia (Hu 1964; Pilger 1903 as ssp. (wallichiana);
however, de Laubenfels (1988) adopted T. sumatrana for his
treatment of gymnosperm taxa in Flora Malesiana. He indicated that
several species may overlap in the eastern Himalayas, suggesting that T.
wallichiana was outside the Flora Malesiana region, but considering
the numerous synonyms and references he provided, one might have also
expected more on distinction between T. wallichiana and T. sumatrana (Miq.) de Laub. Taxus wallichiana has
largely been ignored by Rehder (e.g., Rehder 1940, 1949) and Hortus
Third (Liberty Hyde Bailey Hortorium Staff 1976), while others have
mentioned it as a species confined to the Himalayas (Krüssman 1985), or
more limited to the northwestern Himalayas (Wilson 1926), or as one of
two partially sympatric species predominantly Himalayan in distribution
(Silba 1984). Since Pilger (1903, 1916) did not cite any specimens for Taxus
in western Himalayas, but indicated T. wallichiana to occur in
eastern Himalayas, this omission may reflect uncertainty on his part as
he noted there were intermediates to T. baccata.
I do not accept all morphological variation of Taxus in
southeastern Asia to belong to a single species (Appendix 1). My
interpretation agrees in part with that of Handel-Manzzetti (1929),
Florin (1948a), and Hu (1964). They recognized another sympatric species
by the lack of papillae on the abaxial leaf midrib, which I consider
applicable to a species group typified by T. sumatrana (Spjut
1998b, 2000a, Spjut 2007b). This includes the types of T. mairei, T.
speciosa, and T sumatrana and other undescribed species. However, Kwei & Hu
(1974) and Cheng & Fu (1978) recognized intermediates between T.
wallichiana (papillose midrib) and T. sumatrana (smooth
midrib), and treated the latter as a variety under two illegitimate
combinations (T. chinensis var. mairei, T. wallichiana
var. mairei), whereas Spjut (1993, 1998b, 2000a) reported other
correlative features such as epidermal cells in transverse sections
appearing angular in a Wallichiana Subgroup of species (C & E
Himalayas to SW China; North America) and elliptical in a Sumatrana
Group of species (E Himalayas to Indonesia, Philippines).
The Sumatrana Group is generally found at lower
elevations—on mainland Asia, below 1700 m (Hu 1964), or below 1200 m
(Li & Fu 1997)—whereas T. wallichiana usually occurs above
2300 m. Within the
Sumatrana Group, I distinguish T. mairei by the acute to
obtuse leaves with relatively short trapezoidal, somewhat inflated (mammillose)
epidermal cells on an elevated, truncate to channeled midrib as seen on
the abaxial surface—indicated in my annotations accompanied by crude
illustrations (A, GH, June 1996). Leaves
of T. sumatrana differ by tapering to an
acuminate apex and by nearly rectangular shape of abaxial epidermal
cells. Leaves of T.
sumatrana var. sumatrana are mostly puckered on drying with revolute margins and a
raised abaxial midrib in contrast to those of T. celebica that
are relatively flat with a flush midrib.
Taxus speciosa differs from T. mairei only in
features of branching and color (in dried specimens), treated as a
variety of T. mairei in my annotations. Also, I recognized
another species by conspicuous persistent bud-scales at base of
branchlets, and by leaves that are rigid, evenly tapered to base and
apex (e.g., Fig. 2 in Li 1963), and with a rusty orange color in the
herbarium (T. kingstonii Spjut ined.). Differences
in seed shape and color are also evident among these taxa, but taxonomic
emphasis on seed characteristics could lead to recognizing more taxa.
The taxonomic and ecological significance of midrib papillae on the
abaxial (ventral) epidermal surface of leaves in Taxus was
studied by Bertrand (1874), Cheng & Fu (1978), Deryugina &
Nesterovich (1981), Florin (1931, 1948a, 1948b), von Frimmel (1911),
Kwei & Hu (1974), Orr (1937), and Spjut (1992, 1993, 1998a, 2000a;
Spjut in Hils 1993); however, intermediates have been recognized by
partially papillose midribs. These intermediate usually have elliptical shaped epidermal
cells in leaf transverse sections and are thus considered to belong to
the. Sumatrana Group, in contrast to entirely papillose midribs
of the Wallichiana Group or the entirely smooth midrib cells that
is characteristic of the Sumatrana Group.
Whether these intermediate represent evolutionary, environmental,
or hybrid variants, has yet to be determined.
I suspect all three contribute to variation in leaf anatomy.
Nonetheless, I consider the ancestral type of Taxus leaf to
have stomata distributed completely across the undersurface without
differentiation of an epidermal midrib. The evidence for this is seen by
comparing stomatal features of other taxads with those of Taxus,
and by phytogeographical patterns on numbers of stomata rows within the
genus Taxus (Spjut 2007a).
The stomata in extant Taxus are always encircled by
papillose accessory cells (only partially in T. canadensis), in
contrast to other taxad genera. Austrotaxus, for example, has
hypostomatic leaves that lack papillae and distinct bands, and stomata
are more randomly distributed; Pseudotaxus (Nothotaxus Florin 1931, 1948b)
has 23–28 rows of stomata in bands well defined by glaucous cells
instead of papillose cells, although subsidiary cells are papillose, and
is also ancestral in regard to additional sterile scales in male cones
(Florin 1948b; Miller 1988). Within Taxus, particularly T.
wallichiana and T. chinensis, stomata are occasionally seen
on the epidermal midrib that divides the stomatal region into two bands,
extending almost continuously across the midrib in Kingdon Ward 8594
(K) from Assam, and in Hooker & Thomson s.n. (GH) from Sikkim
(var. yunnanensis), and stomata may also extend to near the leaf
margins in up to 21 rows, whereas in North American plants not more than
11 stomata rows/band have been found (Spjut 1992, 1993, 1998a, 2000a).
Additionally, species that have more conspicuous papillae along cells
walls (e.g., T. brevifolia, T. globosa, T. wallichiana) appear
less variable in the distribution of papillae on the undersurface of
leaves (including juvenile leaves), compared to those species that have
papillae more erect on upper surface of cells (e.g., T. sumatrana).
This may relate to a gradual evolutionary loss of leaf stomata followed
by a reduction in the width of the stomatal region in T. wallichiana,
compared to a possible widening of the leaf by addition of cells within
the marginal region in T. sumatrana.
The presence of papillae on the undersurface of leaves in the T.
sumatrana Group may be of secondary origin—after papillae and
stomata became lost. This is evident by a sharp demarcation between the
stomata bands and adjacent epidermal cells that are variable in
distribution of papillae. For
example, leaves of Wilson 1265 (A, BM, K, S, US) collected from
600–650 m in western Sichuan were found with15–21 stomata rows/band
bordered by a partially papillose marginal region with papillae on 6 of
the 16–25 cells across. Leaves of three specimens from a related plant
grown from seed of Wilson 1265 at the Royal Botanic Gardens in
Kew all lacked papillae entirely along an abaxial margin zone of 18–28
cells across, but consistently had 8–10 stomata rows/band. Other
related specimens cultivated in the United States for which I received
20 leaves (Phyton s.n.)—from apical buds to 3rd yr
branchlets—were found to be relatively constant in the number of
marginal cells without papillae—9 cells across—and also in having
16–18 rows of stomata rows/band, but were variable in shape and length
of epidermal cells, and in the development of midrib papillae,
especially the young leaves. This introduction of Wilson 1265,
apparently from Sichuan near Mt. Emei at 600 m and/or Yachou Fu at 600
m, is not T. chinensis as indicated in the literature (Rehder
& Wilson in Sargent 1914); however, Wilson 1265 was
reportedly collected from three localities (Rehder & Wilson in
Sargent 1914), one of which I have identified a specimen as T.
chinensis—from western Hubei south of “Ichang,” 600–1300 m.
Taxus wallichiana is also interpreted to occur on Mt. Emei in
Sichuan (China) where it integrades with T. chinensis. Subtle differences in size of bud-scales and color of
branchlets make it difficult to consistently separate the two species.
These problematical plants may be hybrids between T. wallichiana
var. yunnanensis and T. chinensis, and/or possibly another
species I recognized by slightly larger and more persistent scales at
base of branchlets (T. scutata Spjut ined, inadnot., A).
Species appearing most related to T. wallichiana include T.
globosa and T. brevifolia in North America (Spjut 1998b) and
two undescribed in Asia. They share character features of conspicuous
bud-scales at base of 1st year branchlets, angular epidermal
cells in leaf transverse sections, leaves densely papillose on the
abaxial midrib with papillae most conspicuous along cell walls, and
seeds maturing on 2nd yr or older branches (Wallichiana Species
Group Spjut 1992, 1993, 1998b; Wallichiana Subgroup, Spjut 2000a,
c). One in Myanmar (T.
suffnessii Spjut ined., type Kingdon Ward 20902 [A, BM]) is
distinct for its relatively large persistent bud-scales (2–3 mm long)
with a conspicuous midnerve. Another in Yunnan and Sichuan (T.
florinii Spjut ined., type from Yunnan, R.C. Ching 21980, A) is much like T. globosa
and T. brevifolia in North America by the large angular epidermal
cells in leaf x-sections, and by stomata developing in less than 12
rows/band; it seems to differ from the North American species by the
thicker walled epidermal cells.
1b. Taxus wallichiana var.
yunnanensis (W. C. Cheng & L. K. Fu) C. T. Kuan, Fl. Sichuan. 2:
215. 1983. Taxus yunnanensis W. C. Cheng & L. K. Fu, Acta
Phyto. Tax. Sin. 13 (4): 86, fig. 52, 4–7. 1975. Taxus chinensis
(Pilg.) Rehder var. yunnanensis (W. C. Cheng & L. K. Fu) L.
K. Fu, Vasc. Pl. Hengduan Mount. 1: 214. 1993. Type: CHINA.
Tibet: Zayul, 2100 m, Zhang 916 (holotype: CAF; isotype: PE-leaf
fragment! photocopy! http://www.cvh.org.cn/pic/pe/0/00000090.jpg). Topotypes: PE (no other data, leaf fragments!],
BM (Kingdon Ward 10398!).
Shrub or tree to 20 m high; branchlets subpinnate, simple to
isodichotomously or isotrichotomously divided, yellowish green, reddish
orange or abruptly reddish purple in 2nd yr, leafy to base;
bud scales persistent, brownish, overlapping in 3–4 ranks, the lower
scales adnate, ovate, ca. 0.5 mm long, upper scales thick, incurved,
spreading, concave, ca. 1.5 mm long. Leaves ± in two ranks, slightly
overlapping in pairs, more evenly spaced along branchlets than in
typical variety, lanceolate, acuminate, mostly straight, rarely falcate,
1.5–3.5 (-4.7) cm long, 2.0–4.0 mm wide, 150–250 µm thick, dark
glossy green above, pale green to yellowish green below (in dried
specimens), slightly convex above to a rounded midrib that forms a
channel along the base on the lower half of leaf, nearly flat below to a
flush to slightly rounded midrib, plane to abruptly revolute 80-90º
near margins; upper (adaxial) epidermal cells mostly rectangular in
x-sect., some cells appearing quadrate, occasionally taller than wide,
25 (-50) µm tall, 25–37 (-50) µm wide; lower (abaxial) epidermal
cells often similar in size to upper in x-sect. as seen near margin,
usually taller than those of var. wallichiana, numbering 7–28
between margin and stomata band, irregularly rectangular, often 3–5×
l/w, becoming longer and
more uniformly rectangular near margins, entirely papillose across the
abaxial surface, or papillae lacking on up to 6
rows across, midrib cells narrow rectangular, 3–7× l/w;
papillae mostly erect, medial in 2–4 opposite rows on midrib cells,
medial on marginal cells; stomata bands broader than marginal region,
with 13–19 stomata rows/band. Male cones maturing on 1st
and 2nd yr branchlets, scales in 4–5 ranks; microsporangia
6–8 (5 fide Cheng), pinkish or brownish. Female cones, 1–2 mm
long in bud, scales in 5–9 ranks, basal scale not conduplicate,
maturing on 1st or 2nd yr branchlets; seed
conical, 4 mm long, 3 mm diam.; sharply pointed at apex, purplish.
Yunnan yew. Distribution: mixed forests types, generally at higher
elevations than var. wallichiana, 2100–3500 m; India (Sikkim,
Nagaland), Myanmar, China (Tibet, Yunnan), occurring with Larix
griffithiana Carrière. and Picea spinulosa (Griff.) A. Henry
in the Sikkim region (Rau 1974). Reported from Sichuan in Spjut
(2007b) based on two specimens cited on this web page that were
erroneously placed here; however, this variety might be expected to
occur in Sichuan.
Representative
Specimens—India—Sikkim:
Terup, 7000–10,000 ft, Hooker s.n., Herb. Hook. fil.,
right specimen (GH); ex Hooker fil & Thomson (BM, GH); Tongloo
[“immense tall tree with long sparse branches and slender drooping
twigs,” “9500-10,000 feet” fide J. Hooker, Elwes &
Henry 1906], Kurz s.n. (A). Nagaland:
Barail Range, Naga Hills, 28º35’N, 93º55’E, 9-10,000 ft, tree,
scattered along summit ridge, Kingdon Ward 7755 (K); “Japuo
Range,” 7300 ft, Kingdon Ward 18990 (BM);
“Jakpho,” “Naja Hill,” 8500 ft, Clarke 41238B (K).
Myanmar
(Burma):
Mt.
Viatoria, 9000-10,000 ft, tree with weeping habit, smooth almost
purplish bark, Kingdon Ward 22819 (BM); Myitkyina Dist., Laikam
Fenshuling Rd, 8000 ft, tree 40-50 ft, Kernode 17205 (K). China—Tibet: Zayul, Rong Tö Valley,
8000 ft, spreading tree with brilliant green foliage, amongst deciduous
trees on slopes and in gullies, Kingdom Ward 10398 (BM);
Delei Valley, 9000 ft, Kingdon Ward 8594 (K); Delei Valley,
Chiban, 28º10'N,
96º30'E, Kingdon
Ward 8090 (K). Yunnan: [Nur ein Baum ober den Tempeln auf
dem] Dji-shan ad boreo-orientem urbis Dali (Talifu), 3200 m, Handel-Mazzetti
6408 (GH); Xangbi Xian, W side of Diancang Shan mountain range,
Malultang, vicinity of Chang Shan, mixed broad-leaved evergreen forest,
2700 m, 25º46' 100º01, 1984 Sino-Amer. Bot. Exped. 388 (GH,
US); W, Shangschang, above Yangbi, 2700 m, tree to 8 m, 1981
Sino-Brit. Exped. To Cangshan 0419 (A, K); Lung-pan la Champu fung,
small tree, 10 ft, forest, fruit gray, C.W. Wang 67412 (A), 67414 (A); Chen-Kang Hsien, ravine, 20 m high,
male, C.W. Wang 72417 (A);
Kiemiu-ingdi above Yangbi, 3000 m, Sino-Brit. Exped., Cangshan
0227
(K).
[Note: Prior to July 2007, two specimens from Sichuan were mentioned
here. These have always been regarded as T. florinii. It
appears that they had been inadvertently placed here].
|
|
 
 
China—Tibet: Zayul, Rong Tö Valley,
8000 ft, Kingdon Ward 10398 (BM), topotype. Specimen
photographed in Oct 1997 (left) and again in April 2005 (right).
The more recent photo shows loss of leaves on lower branch, and an
occasional leaf from other branchlets. Taxus needles
can be
easily dislodged if specimens are not handled
with extreme care. Loans sent out should enclose each specimen
in a sheet of paper that completely covers the specimen. One
loan I received had papers only partly covering each specimen;
consequently, the paper would clip leaves as the specimens were
moved. The
leaves of var. yunnanensis from the type locality are much
like typical T. wallichiana in the furrow on the
darker adaxial surface; however, the leaf in
x-section appears relatively thin and plane. Another
difference is the abaxial leaf surface has many rows
of enlarged marginal cells, 3 of which are smooth and 8 of which are
papillose. The papillae are opposite (and medial), appearing more
distant in the type than in other specimens.
The marginal cells are followed by 14 rows of stomata. |


China—Tibet: Delei Valley,
Chiban, Kingdon
Ward 8090 (K). This specimen appears
similar to the type. Younger leaves appear
more furrowed (when dried) on the adaxial surface; the leaf
section—from an older leaf—is not furrowed. As in the type,
the abaxial leaf surface lacked papillae on three marginal cells and
was followed by 8 papillose cells and 14 stomata rows. The
midrib cells appear narrower. Papillae are distinctly medial,
in a single file on narrow cells, and in opposite rows on wider
cells. The leaf mesophyll is illustrated to have a reticulate
arrangement of bone-like parenchyma cells with rounded intercelluar
spaces. |
 

China—Yunnan: Shangschang, above Yangbi,
2700 m, 1981
Sino-Brit. Exped. to Cangshan 0419 (A). Top left specimen with
lower color photo shows reddish midrib and greenish stomata band.
Observe that papillae are marginal but opposite, such papillae are
usually medial. This character attribute is intermediate to
T. chinensis. Observe also the flattened (plane) leaves,
appearing lanceolate in shape. This is in contrast to var.
wallichiana that has strongly curled leaves, appearing
compressed laterally so that the adaxial midrib is furrowed.
Observe also dark persistent bud-scales at base of branchlets in
lower photo and seed on 2nd yr branchlets in upper right photo.
|

China—Yunnan: Dali (Talifu), 3200 m, Handel-Mazzetti
6408 (A). Three color prints and packet with author's sketch are
attached to this specimen. This specimen differs from the typical
form in the leaf shape, which is more like T. chinensis than
T. wallichiana. The dark colored branchlets, the persistent
scales at base of branchlets, and the large red epidermal cells—as shown
in lower photo (25 µm tall and wide)—are
characteristics of T. wallichiana. The medial opposite
papillae on the abaxial midrib—as seen in the top left photo—further
differentiates this as T. wallichiana var. yunnanensis. The top
right photo shows a stomata band that is also much like T.
chinensis, and marginal cells enlarged only in several
rows; the packet sketch indicates 7 marginal cells followed by 13
rows of stomata. |
|
 
India-Nagaland:
Barail Range, Naga Hills, 9000-10,000 ft, Kingdon Ward 7755
(K). This specimen differs from the typical form in the lack
of enlarged epidermal cells along the abaxial leaf margin.
Otherwise, it agrees with var. yunnanensis in shape of leaf
in x-section, in having 4 rows of smooth marginal cells
followed by 14 rows of papillose cells and in the 14 rows of
stomata. The papillae were noted to be opposite and very
prominent, even occurring in two rows on narrow midrib cells. |
  India-Nagaland:
“Jakpho,” “Naja Hill,” 8500 ft, Clarke 41238B
(K). This specimen differs from the typical form by the paler
orange instead of dark purplish colored branchlets. The leaves
appear similar to the type in shape and anatomy. The abaxial
leaf margin was noted to have 3 rows of smooth cells followed by 13
rows of enlarged papillose cells, 11/12 stomata rows, and 12 rows of
rectangular midrib cells. As in the type, the papillae appear
relatively small in size and distant and opposite each other.
The leaf mesophyll is shown to have a reticulate network of
parenchyma cells with rounded intercellular spaces; in the upper
right hand corner is a more detailed drawing of the parenchyma cells
removed from the mesophyll region.
|
 
Myanmar: Myitkyina Dist., Laikam
Fenshuling Rd, 8000 ft, Kernode 17205 (K). This specimen
has leaves shaped more like specimens from Yunnan than from the type
locality in Tibet. However, the leaf epidermis is much like
the type; the abaxial surface has 3 rows of smooth cells followed by
10 rows of enlarged papillose cells and 12 stomata rows. The
papillae are medial, opposite and distant. The mesophyll
parenchyma cells appear to represent a distinct pattern not seen in
other species, but no taxonomic distinction is made for leaf
mesophyll here as this character is often difficult to evaluate in
dried specimens. Also noted is the sharp contrast in color
between the red adaxial epidermal cells, and yellowish to yellowish
green stomata and yellowish abaxial midrib. |


China—Tibet:
Delei Valley, 9000 ft, Kingdon Ward 8594 (K). This specimen
differs slightly in the epidermal cells not being sharply
quadrangular. The abaxial leaf surface was noted to have an
olive green color with little differentiation in midrib,
margin and stomata band regions. The upper right corner of the
packet shows a sketch of the bone-like mesophyll parenchyma cells. |
|


India—Sikkim:
Terup, 7000–10,000 ft, Hooker s.n. (GH), packet illus below
drawn from specimen at K. This specimen is remarkably similar
to the one above collected by Kingdon Ward 7755 from
Nagaland.
|


China—Yunnan: Diancang Shan mountain range,
Malultang, vicinity of Chang Shan,
2700 m, 1984 Sino-Amer. Bot. Exped.388 (GH). |
 

China—Yunnan:
Kiemiu-ingdi above Yangbi, 3000 m, 1981
Sino-Brit. Exped. 227 (K) |

China—Yunnan: Chen-Kang Hsien, ravine, 20 m high,
male, C.W. Wang 72417. Intermediate: Papillae noted be marginal
on midrib cells, but appearing medial on marginal cells. Treated
previously under var. wallichiana. |
| |
  
T. yunnanensis, photocopy
and photo of isotype at PE |
|
|
Taxus yunnanensis has been confused with T. wallichiana
in the Flora of China (Cheng & Fu 1978). The authors had
evidently considered the type for T. wallichiana to represent the
species mainly in NW Himalayas; consequently, they described T.
yunnanensis—indicating it was found in eastern Himalayas (Bhutan,
Tibet, Myanmar) to Yunnan and Sichuan (Cheng et al. 1975; Cheng & Fu
1978). Later, it was reduced to a variety of T. wallichiana as
cited above, and more recently placed in synonymy (Li & Fu 1997),
although it had been included in synonymy by de Laubenfels (1988) under
his broadly circumscribed T. sumatrana.
Most specimens I annotated T. yunnanensis (A, GH, July 1996;
BM, Oct. 1997; Spjut 1998b) were from Yunnan and Sichuan. They were
distinguished from typical T. wallichiana by the leaves appearing
slightly wider (nearly lanceolate), more evenly distributed, less
markedly curved above and paler green below than above, and having
medial papillae on the abaxial epidermal cells.
At the time I had seen only leaf fragments of a type—from Tibet
near the border with Myanmar and India; it differed from the type of T.
wallichiana by the abaxial surface having a broad region of large
epidermal cells with medial papillae between the margins and stomata
bands. These features were seen more often in yew specimens from Yunnan
and Sichuan than in those from northeastern India. Later, I received a
B/W photocopy of a PE isotype from Dr. Z-y. Cao who had earlier sent me
leaf fragments of topotypes, and found that the leaf arrangement and
shape compare closer to the type of T. wallichiana than to
specimens from Yunnan and Sichuan. Thus, plants most typical of this
variety as seen in northeastern India and nearby Tibet are intermediate
forms, distinguishable only by leaf anatomical characters. For this
reason T. yunnanensis is reduced to a variety.
Nevertheless, it is important to differentiate these and one
other related species under study (T. florinii Spjut ined) in
order to distinguish the North American species (T. brevifolia, T.
globosa) from their Asian relatives; otherwise, they would have to
be included under T. wallichiana.
2.
Taxus contorta Griffith, Not. Pl. asiat. 4: 28. 1854 (“Taxus contortus?
Vide Itinerary Notes, p. 351, No. 116”.
See also Itin. pl. Khasyah mts., II: 351. [1847-]
1848 [Book III,

Chapter II, “Affghanistan Flora, Second year
Kafiristhan.116. Taxus?” “Arbor, foliis alternis linearibus
compressis, sulcato univeniis basi ½ tortis. Brought from Kafiristhan
with the preceding [Pinus], the undersurface of the leaves
subsequently becomes uppermost from torsion of the base. The change
takes place gradually judging from the slight obliquity of young leaves.
Stomata blocked up, with a brown curious cuticular substance.”]). TYPE: AFGHANISTAN. W of Kabul, “Bharowul, in woods, 7000–7500
ft” (locality data from Griffith nos. 112-114 in Itin. pl. Khasyah mts,
collected during 1839–1841), Lectotype—Griffith 5002 at K! (lower right
specimen of three on one sheet, Fig. 11, the other two specimens
evidently part of a separate single collection with handwritten note on a label
indicating bark was used in a tea in Ladakh, det. by Spjut as T.
contorta, designated by Spjut 2007). Note that both publications by Griffith have to be
applied together for valid publication. used in a tea in Ladakh, det. by Spjut as T.
contorta, designated by Spjut 2007). Note that both publications by Griffith have to be
applied together for valid publication. |
|
 
Photocopy of relevant pages from Griffith, Notulae Plantas Asiaticus,
1854. |
Taxus orientalis Bertoloni,
Mem. Acad. Sci. Bologna ser. 2, I, 229, pl. 2 (1862); Misc. bot. 2 3:
17, Tab. 2 (shown here). 1862, and holotype. 3:
17, Tab. 2 (shown here). 1862, and holotype.
|
|
Taxus fuana
Nan Li & R. R. Mill in Li & Fu, Notes on gymnosperms I. Taxonomic
treatments of some Chinese conifers. Novon 7: 263. 1997 (Nov.).—TYPE:
CHINA. Tibet (Xizang): Jilong, 3000 m, Qingzhang Expedition 7032; holotype
PE!
 Taxus orientalis Bertoloni,
Mem. Acad. Sci. Bologna ser. 2, I, 229, pl. 2 (1862); Misc. bot. 23: 17,
Tab. 2. no specimens cited. TYPE: northeastern India, western Sikkim,
8,000 ft (Holotype: BOLO [leaf fragments!]).
 |
|
2a. var. contorta.
Ultimate branchlets yellowish green, gradually reddish or yellowish
brown; bud-scales persistent or semi-persistent at base of current
growth in 3–4 ranks; these thick, greenish to brownish, the lower
scales paleaceous, ovate or deltoid, concave, acutely folded along a
prominent midnerve, ca. 0.5–1 mm long, the uppermost cucullate, ca. 1
mm. long. Leaves closely overlapping, linear and sharply apiculate,
straight to falcate, 1.5–3.5 cm long, mostly ca. 2 mm wide, 350–500
µm thick, olivaceous and convex above to a rounded midrib that forms a
slight channel along each side at base, olivaceous or yellowish green
and plane to slightly concave below to a flush or slightly rounded
midrib, abruptly revolute near margins, or only slightly revolute near
margins; upper (adaxial) epidermal cells in x-sect. elliptical to
slightly angular in plants from Nepal (probably hybrids with T.
wallichiana), 10–15 µm tall and 20–35 µm wide; lower (abaxial)
epidermal cells quadrate in up to 6 rows nearest margins, becoming long
fusiform to long rectangular towards stomata bands, not always distinct
from those in stomata bands, 3–10× or more l/w, 8–12 µm tall,
10–25 µm wide, prominently papillose to within 4–6 (-8) cells of
the margins; stomata bands greenish, or yellowish green, narrower than
the marginal region, with (5-) 7–8 (-9–11) rows of stomata, the
stomata counts generally lower in the NW Himalayas and higher in Nepal;
palisade parenchyma of 1 row, 50–70 µm long; spongy parenchyma cells
elliptical, resinous, with sclerified walls, loosely connected, falling
apart when leaves of herbarium specimens are soaked and sectioned. Male
cones abundantly produced near ends of branchlets, their buds globose,
ca. 2 mm diam. Female cones maturing on 1st and 2nd
yr branchlets; seeds subcylindric to obconic, ca. 6 mm long, 4 mm diam.,
abruptly tapering near apex.
West Himalayan yew. Distribution: Mixed
coniferous-hardwood forests of W Himalayas, 2300–3500 m; Afghanistan,
Pakistan, India, W Nepal, China (SW Tibet). Noted to be common in the Garhwal and
Kumaon regions at elevations near 8500 ft (Gamble 1922; Gordon 1875)
where clouds often hang in oak-conifer forests of Quercus
semecarpifolia Sm., Abies pindrow (D. Don) Royle, and Rhododendron
arboreum Sm. (Freitag 1971; Rau 1974). In the Uri Range closely
associated with Abies pindrow-Picea smithiana (Wall.) Boiss.
forest (Sapru 1975), a vegetation type common to the higher ranges in
the W and C Himalayas of India and Nepal (Champion & Seth 1968; Rau
1974). In the Kumaon and Nepal regions mostly on N side of the Himalayas
in hemlock (Tsuga dumosa (D. Don) Eichler) forests with spruce (Picea
smithiana) as a common associate (Rau 1974), especially near Rara
Lake in W Nepal (Stainton 1972).
Representative
Specimens—Pakistan:
Between Gotchbok and Kubkot Valley, 2750 m, Sinnott et al. 146
(K); Punjab Province, near Rosenhiem, Murree, Rodin 5313 (US);
Murree, 7000 ft, Stewart 15343 (NA, US), Sprague 730 (K). India—NW
Himalaya: Punjab, Kulu, above Bandrole, elev. 8000 ft, Koelz
10285 (A, NA); near Kulu, Bushreo Pass, Koelz 3119 (US); above
Jaurah, Tehri, Koelz 10385 (NA); Kashmir, Pahlgam, 7000–10,000 ft,
tree, Stewart 5931 (A), 8414 (A, NA, PH, US), 12001B
(A), 2600 m, Heybrook 29 (K); Kashmir, Sukhi across the Bamsuru
and Chaia Pass to Khdrsali (Passes between the Bhagiratti and Jamma
Valley), 9000–15,400 ft, Schlagintweit 8941 (GH); Kashmir, Lida
Valley, Mukiji (K); Bashahr, Uri Forest, 20 Jun 1890, J.H.
Lace 301 (A); NW of Srinagar, ex Herb. Schlagintweit s.n.
(PH); Sonamarg, 10,000 ft, Stewart 7374 (PH); Gulwarg,
7000–10,000 ft., Stewart 10663A (PH); Chamba, Kalatop Reserve,
Pergamma Bathri, 8500 ft, 12–4-1920, R. N. Parker s.n. (A);
Dharmkat, Dharmsala, Stewart 1938 (BH, PH); Siwalik and Jaunsar
Div., 10,000 ft, Laig Raus (P); Baltal to Nunner, ex Herb.
Schlagintweit 4795 (P); Kujiar, Chunuba, 7000 ft, 2 Oct 1874, C.B.Clarke
s.n. (A, P); Chumba, Pengelly s.n. (K); Panwanle Kanta, 9500
ft, Sahni 21664 (BH), 21669 (BH); Bureah, 11,000 ft, Kenyoer
& Dugeon s.n. (PH); Tehri above Jaurah, Koelz 22084 (NA);
Garhwal to Lake Hemkund, 3200 m, Rau 31770 (A); Kumaon, Wallich
6054/B (BM on three sheets, K, p.p.; M, p.p.; P on three sheets,
p.p.; K, M & P also have Wallich specimens of T. contorta
numbered 6054A); Kumaon, Dwali? 8500 ft, ex Herb. Falconer
1000 (GH, P, S). Matiana Hill, ex Herb. Reg. Bot. Calcutta (BH).
Nepal: Dhotar, 9600 ft., Polunin et al. 1353 (BM),
Chankeli Range, 8000 ft, Polunin et al. 432 (BM); W of Jumla,
Belas Gaejigeth, 10,000 ft, Polunin et al., 5050 (BM); Lete, S of
Tukucha, 8000 ft, Stainton et al. 734 (BM); Chingnon, N of
Tukucha, Gadaki Valley, 10,000 ft, Stainton et al. 7832 (BM);
5616 (BM); Dhaulagiri Zone, 2405 m, Mikage et al. 9550282 (BM);
Marayandi Khola, Ottba et al. 8311066 (BM). China Tibet
(Xizang):
Gyirong County Bangxing, 3000 m, Li-Chen Shu-kun, Du 589 (PE);
Gyirong County, Shenyang, 3100 m, Tibet Team 13 (PE); in several
villages Gyirong County District Gilon, Jisonglin geopolitical hillside
fire, 3000 m, Qinghai-Tibet Team 7032 (PE, evidently an isotype
but not indicated as such). Note: citation of PE specimens from
virtual online herbarium, spelling as it appeared on that site.
Additional material (needles) received by Spjut from Dalian Inst. Chem.
Physc, 30 Apr 2007, reportedly collected from JiLong, 2750 m, JiLong
Forestry Bureau staff expert (CAS, wba [needles]).
|
  India—NW
Himalaya: Left—Punjab, Kulu, above Bandrole, 8000 ft, Koelz
10285 (A).
Himachal Pradesh. Right—Bashahr, Uri Forest, 20 Jun 1890, J.H.
Lace 301 (A). These specimens are typical of the species in
the distichous arrangement of the leaves spreading mostly ~45º.
Branchlets appear to be pendulous. The most distinctive
character feature is the rounded parenchyma cells is the leaf
mesophyll. These often appear like red egg shells. They
are loosely contained within the mesophyll, and fall out when
leaves are sectioned. |
 

Nepal: Chankeli Range, 8000 ft, Polunin et al. 432 (BM).
Left—specimen typical of the species, right—close-up of branchlet
showing scales, mostly in a single series at base of branchlets,
often not persistent; det. T. contorta by Polunin et al..
|
 
 
Nepal: Dhotar, 9600 ft., Polunin et al. 1353 (BM).
Differs slightly from the typical form in that leaves spread more at
60º;
annotated T. angustifolia by Franco. |


India: Jaunsar Dist., 10,000 ft.,
Gamble 23507 (K). In this specimen leaves appear more distant
and radial in arrangement. |
 

India—Siwalik
and Jaunsar
Div., 10,000 ft, Laig Raus (P). |
 
India: Kumaon, Dwali? 8500 ft, ex Herb. Falconer
1000 (GH). |
 
Kashmir, Pahlgam, 7000–10,000 ft,
tree, Stewart 8414 (A). |
 
India—Chamba, Kalatop Reserve,
Pergamma Bathri, 8500 ft, R. N. Parker s.n. (A); annotated T.
orientalis. |
|
 
India—Tehri above Jaurah, Koelz 22084 (NA). |

Wallich s.n. & locality data, with T. wallichiana
(P). |
 

Pakistan:
Between Gotchbok and Kubkot Valley, 2750 m, Sinnott et al. 146
(K), annotated T. wallichiana by Farjon (Mar 1996). |
 
India: Chumba, Pengelly s.n. (K); annotated T.
fuana by Fu & Li Nan. |
|
Taxus contorta is one of the easiest yew species to identify.
The relatively long, straight, narrow leaves are generally crowded along
stems in nearly two ranks that in the typical form generally do not
spread more than 60° from branchlets. The leaf mesophyll contains
distinctive parenchyma cells (idioblasts) that appear reddish in dried specimens
(after soaking in water). These cells occur predominantly across the mid region of the
leaf mesophyll and around the diffusion area of the vascular bundle.
They are not sclerenchymatous, but appear the reddish striations
appear to be
resinous deposits on cells walls.
Rao and Malaviya (1965) described what they called
“osteo-sclereids” in leaves of one of four varieties of T.
baccata they reportedly studied; however, their material reportedly
lacked cones, and their illustrations of leaf sections showing sclereids
are reminiscent of what I have observed in Asian species of
Torreya.
Taxus baccata has similar idioblasts as seen by the
spherical shape and dark color, but do not show the sclerified
(striated) walls, and do not fall apart when sectioned.
The Cuspidata Alliance generally
has a leaf mesophyll largely of loose spherical to ellipsoidal cells
connected together by short cylindrical cells that are without
idioblasts.
Taxus contorta is more related to T. baccata (lectotype
based on “Hort. cliff. 464”; Jarvis et al. 1993, Clifford
Herb., BM!) than to T. wallichiana by the relatively low number
of leaf stomata rows/band—usually 7–8, by the ellipitical epidermal
cells as seen in leaf x-section, and by characteristics of leaf
mesophyll cells as just described. The leaf mesophyll of T.
wallichiana in contrast usually has periclinally oriented, elongate
cells connected in a skeletal-like net; in longitudinal sections these
cells do appear like bones. Additionally, T. baccata exhibits
more variation in Europe than T. contorta in the Himalayas in
branching, leaf arrangement and leaf anatomy. Of particular relevance is
the occurrence of papillae on the abaxial midrib of leaves—that in T.
baccata can be densely papillose (e.g., lectotype) or entirely
smooth (e.g., Curic s.n., from Bosnia, K), whereas T. contorta
always has a densely papillose midrib.
The close relationship between the European T. baccata and
Himalayan T. contorta was recognized by Handel-Mazzetti (1929)
and by Florin who in his annotations of specimens at Stockholm (S)
regarded it as a subspecies of T. baccata in which he had adopted
the epithet from T. orientalis Bertol., a later name.
Other botanists have independently recognized T. contorta as
distinct from T. baccata and T. wallichiana, but by names
that are not always in accordance with the ICBN. For instance,
Handel-Mazzetti (1929) correctly realized that T. wallichiana was
based on Wallich 6054A, but referred the northwestern Himalayan
yew to T. orientalis. He
was also aware of T. contorta, which he referred to as a nomen
nudum; however, Griffith (1854) referred back to his earlier 1848
publication as referred to above; thus, T. contorta is not a
nomen nudum (Art. 32.3, 32.4, 34.1), and predates T. orientalis Bertoloni
(1862). Franco (1964), who reviewed Taxaceae for Flora Europaea,
also recognized the west Himalayan yew (T. contorta) as a
distinct species, but he annotated specimens (BM) by another name (T.
angustifolia Franco, ined., dated 1956) that if published would have
been illegitimate; more recently, Nan Li & R. R. Mill (Li & Fu
1997) reached a similar conclusion, but did publish their superfluous
name, T. fuana. Occasional collections by Polunin et al. (e.g.,
No. 432, BM) had been correctly determined, while most herbarium
collections of this species have been misidentified T. wallichiana.
It is not clear to what extent Wallich and Griffith had distinguished
yews in the Himalayas. Wallich’s (1826) Tentamen Florae Nepalensis recognized
only one species, determined as T. wallichiana by Zuccarrini
(Siebold & Zuccarini 1843); however, Wallich specimens of Taxus numbered
6054, differentiated by letters A-E, suggest they were
distinguished—at least by collectors and location, and may include an
annotation T. virgata Wall.
(nomen nudum), which I have identified as T. wallichiana (Blinkworth
s.n. BM, reportedly from Kumaon, but probably from Nepal), or T.
baccata (young shoot on sheet with 3 other specimens of T.
contorta, Wallich 6054, ex Herb. Gordon, with “b”
indicated lightly in pencil, K; probably added for comparison).
Most Wallich 6054A (from Nepal) belong to T.
wallichiana, while most Wallich 6054B (from Kumaon) are T.
contorta. Griffith, who worked with Wallich on occasion (Burkhill
1965), also assigned mixed collections of Taxus to the same
number with different data; the type, for example is from Afghanistan,
but other labels with this number (Griffith 5002) indicate the
specimens were collected in the eastern Himalayas. Griffith (1854)
recognized possibly two species from Bhutan, distinguished by
“axillary” and “terminal” “inflorescences” and “3. Taxus
contortus?” by reference to his collection from Afghanistan. I
have noted that three species of Taxus are represented in
Griffith collections.
Similarly, J. D. Hooker may have considered there were three species of
Taxus in India as evident by a specimen in the Gray Herbarium
with three different species (T. contorta, T. kingstonii, T.
wallichiana) all on one herbarium sheet (J. D. Hooker 77, 87,
GH).
Although T. contorta appears quite distinct from T.
wallichiana, hybrids seem evident by the respective higher and lower
counts of stomata rows where geographical ranges of these species
overlap—in central and east Himalayas (Spjut submitted).
This includes the type for T. orientalis (BOLO!)—from
Sikkim. In several leaves
studied of the T. orientalis type, the parenchyma cells were
found to be those of the T. contorta type, whereas the slightly
larger epidermal cells (20-25 µm tall, 25–35 µm wide) along with the
higher stomata counts (10–11 rows/band) indicate affinity to T.
wallichiana, whereas the absence of bud-scales at the base of
branchlets, and the strongly revolute linear leaves (illustrated by
Bertoloni, 1862) are other features I associate with T. contorta.
Specimens of T. wallichiana var. yunnanensis from
Sikkim were found to have stomata in 13 rows (Kurz s.n. A), or 14
rows (e.g., J. D. Hooker & Thomson A, GH, K).
2b. Taxus contorta var.
mucronata
Spjut, J. Bot. Res. Inst. Texas 1(1): 256. 2007. Taxus mucronata Spjut ined. (A, BM)—Type: BHUTAN
(Eastern). Ha: 27º22' 89º18', 9,000 ft, tree 15 ft—11 Apr 1949, Ludlow
et al. 16035, with male cones (holotype A! designated June 1996;
isotype BM! in adnot., Oct. 1997).
Tree to 3 m or more; leaves sharply bent at base of blade, 1.5–2.5
cm long, ca. 2 mm wide; abaxial epidermal cells up to 23 across the
margin, irregularly quadrate in up to 6 rows nearest the margin,
becoming long fusiform to rectangular towards the stomata band and on
midrib, irregular in width, mostly 3–7× l/w, papillose on more than
half of the marginal cells—to within 8 (-5) cells across from margins
and entirely on midrib, the papillae positioned medially in 1–2 rows
on each cell; stomata bands greenish, or yellowish green, narrower than
the marginal region, with 9–11 rows of stomata; palisade parenchyma of
one long row of cells and a much shorter second row; spongy parenchyma
cells nearly globose, many sclerified and resinous. Male cone scales
4–5 seriate; sporangiophores 8, united into a ribbed column ca. 2 mm
long, thickened at apex of column, separating into 8 umbrellalike
segments, each with 5–8-cuculately lobed microsporangia ca. 1 mm diam.
Seed in one specimen globose, reddish.
Mucronate-leaved yew. Distribution: Upper forest region, 2300–3100
m; Nepal, Bhutan.
Additional
Specimens—Nepal:
Dobremez 2106 (BM); Marayandi Valley, 3100 m, Wraber 514
(BM); ridge S of Bhahwe Sekh, 9000 ft, Polunin et al. 1873 (BM);
Dhawalagiri Zone, Mustang Dist., Ghasa, 2405 m, Mikage et al. 9550282
(BM).
|
|

Taxus contorta var. mucronata.
Illustration in Cheng et al. (1975) and Cheng and Fu (1978) that was
referred to as Taxus wallichiana; however, in Li & Fu (Nov.
1997) this was renamed to T. fuana Nan Li & R. R. Mill along
with other specimens cited as “paratypes” from the NW Himalayas that
correspond to var. contorta. In the present paper, T.
fuana is reduced to a
synonym of T. contorta. In China it is reported only from Jilong Xian in SW Xizang (Tibet).
This illustration, reportedly drawn by Liu Chunrong was thought to
be similar to the type, but it is not. The illustration was redrawn in the later
edition of the Flora of China.
|
 
 
Taxus contorta var. mucronata.
Bhutan: Eastern: Ludlow
et al. 16035. Holotype for Taxus mucronata (A),
designated by Spjut in June 1996. Photo of illustration
attached to specimen.
|
 
Taxus contorta var. mucronata.
Bhutan: Isotype
for Taxus mucronata at BM showing annotation label by Spjut,
dated 21 Oct 1997.
|
 

Taxus contorta var. mucronata. Nepal:
Karayandi Valley, Hanang, between Pinang and Chaug, 3100 m,
Wraber 514 (BM). A temporary label was provided indicating
a leaf fragment was taken. The species status of this taxon
was based on a combination of features—sharply reflexed leaves,
relatively short leaves, a relatively wide marginal region of cells on abaxial
surface, and higher stomata count (compared to other specimens from
NW Himalayas)—in only one specimen from Bhutan (GH: holotype).
However. another specimen from Nepal at BM was found to have these
same features, including the marginal cells,
parenchyma cells, and stomata count. |
|
 
Taxus contorta var. mucronata.
Intermediate Nepal: Dhawalagiri Zone, Mustang Dist., Ghasa, 2405 m, Mikage et al. 9550282
(BM). This specimen differs from the Bhutan specimens above in having a
relatively narrow abaxial leaf margin, 4 cells wide,
and by leaves tapering to an acute, apiculate apex.
|


Taxus contorta var. mucronata. Nepal:
Dobremez 2106 (BM). |
 

Taxus contorta var. mucronata
Intermediate
Nepal:
9,000 ft. Polunin et al. 1873 (BM, det. by Polunin et al. as
T. contorta. The relatively longer leaves of this specimen, appearing
more acute near apex (less abruptly tapered), suggests var.
contorta, whereas the conspicuous mucro agrees with var.
mucronata.
|


Taxus contorta var. contorta
Intermediate
Nepal: 8500 ft, Gardner s.n. (BM). The
relatively long
leaves of this specimen favors var. contorta whereas
the wide divergence from branchlets agrees more with var.
mucronata. |
|
I annotated one specimen from the Arnold Arboretum Herbarium, Taxus mucronata
Spjut (ined.) in June 1996, and also designated it as type. Other
specimens at the Museum of Natural History in London (BM) were later
discovered and similarly annotated (Oct. 1997). Although Taxus fuana
was not described until later (Nov. 1997), all specimens I saw also at K
bearing the annotated name T. fuana (by Li and Mill) belonged to
var. contorta, not var. mucronata as I have recognized it
here, whereas specimens I annotated T. mucronata Spjut ined. were
not annotated by Li and Mill. However, the illustration in Cheng et al.
(1975) and in Cheng and Fu (1978), which I had erroneously referred
to as drawn from the type from Tibet, referred to by Li
and Mill (in Li & Fu 1997), undoubtedly belongs here even though Nan
Li and R. R. Mill in my opinion did not distinguish this taxon, but it
is not a type as I have recently discovered; therefore, T. fuana
is now treated as a synonym of T. contorta var. contorta.
All other (eight) specimens they cited, referred to as “paratypes” (from
India, Kashmir, Pakistan, Nepal) belong to var. contorta. Farjon (1998)—by listing the
species as known only from the type locality—excluded T. contorta var.
mucronata. However all material I have seen from Tibet on
the PE website belongs to var. contorta as might be expected since Fu et
all. (1999) report the occurrence of T. contorta in China (as T.
fuana) only in SW Tibet.
This variety is distinguished from the typical variety by the
relatively shorter and more sharply reflexed and abruptly apiculate leaves.
Other differences are seen on the abaxial leaf surface having a smooth marginal border of
5–8 irregularly quadrate epidermal cells, and 9–11 stomata
rows per band; however, occasional plants from Nepal are
difficult to assign to either variety, which is why it is regarded only
as as a variety. A
geographical
analysis of character traits of Taxus contorta revealed that
plants with more widely spreading leaves, and with more stomata and
wider leaf margins, occur in the eastern range of the species where it
is sympatric with T. sumatrana and T. wallichiana.
Also, where these species appear sympatric, T. wallichiana was
found with lower stomata counts. Thus, it would appear that the
merging of these species characteristics is the result of introgression.
However, it might be added that the shorter and more sharply reflexed
leaves are features that are related to T. chinensis and T.
umbraculifera.
|
|
 3.
Taxus chinensis (Pilg.) Rehder, J. Arn. Arb. 1: 51. 1919. Taxus
baccata L. [ssp. cuspidata (Siebold & Zucc.) Pilg.]
var. chinensis Pilger, Pflanzenreich IV, 5: 112. 1903. Taxus
cuspidata Siebold & Zucc. var. chinensis (Pilg.) C. K. Schneider ex
Silva Tarouca, Freiland-Nadelgehölz. 276. 1913. Taxus wallichiana
Zucc. var. chinensis (Pilg.) Florin, Acta Hort. Berg. 14, 8: 378.
1948. Type: CHINA. E Sichuan: Wushanhsien, 2000–3000 m—selected
partially by Rehder & Wilson in Sargent, Pl. wilson. 2: 8. 1914,“Henry
7097, 7155, type,” and by Florin (1948a, l.c.), “Henry 7155”;
here further clarified by specimens in Harvard University Herbaria, 2
sheets, one in A indicated as “type” by Hu, another in GH annotated
as “isotype” by Hu. —Lectotype: sheet in A!
bearing accession # 18682, with seed (isolectotypes: BM! E, GH! K! (Fig.
12) S [fragment], US!). Other
original material 3.
Taxus chinensis (Pilg.) Rehder, J. Arn. Arb. 1: 51. 1919. Taxus
baccata L. [ssp. cuspidata (Siebold & Zucc.) Pilg.]
var. chinensis Pilger, Pflanzenreich IV, 5: 112. 1903. Taxus
cuspidata Siebold & Zucc. var. chinensis (Pilg.) C. K. Schneider ex
Silva Tarouca, Freiland-Nadelgehölz. 276. 1913. Taxus wallichiana
Zucc. var. chinensis (Pilg.) Florin, Acta Hort. Berg. 14, 8: 378.
1948. Type: CHINA. E Sichuan: Wushanhsien, 2000–3000 m—selected
partially by Rehder & Wilson in Sargent, Pl. wilson. 2: 8. 1914,“Henry
7097, 7155, type,” and by Florin (1948a, l.c.), “Henry 7155”;
here further clarified by specimens in Harvard University Herbaria, 2
sheets, one in A indicated as “type” by Hu, another in GH annotated
as “isotype” by Hu. —Lectotype: sheet in A!
bearing accession # 18682, with seed (isolectotypes: BM! E, GH! K! (Fig.
12) S [fragment], US!). Other
original material (syntypes): Henry 6913, Farges 128.
(syntypes): Henry 6913, Farges 128.
Taxus baccata L. var. sinensis, Henry, Elwes & Henry,
Trees Gr. Brit. and Irel. 1: 100. 1906. Type: CHINA. Same locality as
for T. baccata var. chinensis; lectotype here designated: Henry
7097
at E. Isolectotypes: A!
BM! P! US!
Nomen illegit. (ICBN Art. 53.3, Ex. 9). |
| Ultimate branchlets yellowish
green, gradually yellowish red, or yellowish brown, or dull reddish
orange with age; bud-scales occasionally persistent on 1–2nd
yr branchlets, wart like or squamulose, not overlapping, or overlapping
only slightly, ca. 0.3–0.8 mm long. Leaves mostly green, or turning
reddish when dried, narrowly to broadly oblong, 1–2 (-2.5) cm long,
1–3 mm wide, 0.25–0.5 mm thick, strongly convex above to a rounded
midrib, plane to slightly concave below to a rounded midrib; upper (adaxial)
epidermal cells in X-sect. usually elliptical, occasionally angular,
typically wider than tall, 10–25 µm tall and 25–40 µm wide,
occasionally 25–30 µm tall and 35–40 µm wide, thick-walled, not
inflated; ventral (abaxial) epidermal cells not as tall as those on
upper surface, reddish, 5–12 µm tall and 15–25 µm wide, short to
long rectangular, 1–5 (-7)× l/w, more irregularly quadrate near
margins, usually papillose entirely across midrib, and to within 4–12
cells from margins; papillae egg-shaped, positioned marginally (mostly
along cell walls) in 2–3 alternate rows, or occasionally medially on
midrib; stomata in yellowish green to yellowish orange bands broader
than adjacent marginal region, 11–19 (-21) rows/band; palisade
parenchyma cells usually of 1 row, or 2 rows on young leaves, 50–150
µm long. Male cones ovoid in bud, 2–3 mm wide and 4 mm long, maturing
on 1st–2nd yr branchlets. Female cones
subcylindric, to 2 mm long in bud, maturing on 1st–2nd
yr branchlets; seeds subglobose, or conical, slightly angled, to 5 mm
long and 4 mm wide, tapering to apex from mid region.
China yew. Distribution: Forest, or forest margins, or open scrub,
“under rocky cliffs,” “often among bamboos,” generally
1000–2800 m, mostly China (Guangxi, Gansu, Yunnan, Sichuan, Guizhou,
Hubei, Anhui, Zhejiang), one collection from Vietnam. Reported also at
elevations as low as 150 m (Hu, 1964). In Sichuan found more in the
drier “mixed mesophytic forest” or “transitional zone” to an
evergreen oak forest, in contrast with T. wallichiana occurring
more in hemlock-spruce-fir forests (Wang 1961).
Representative
Specimens—Vietnam:
Hoa Binh, Mai Chu, P Co, 900–1500 m, with Podocarpus and Pinus,
Hiép & Chan 405 (P). China—Sichuan: NE,
Tschen-kuu-tin Dist. (Chenkouting, Florin 1948a), Farges 128 (K,
P); Tschsianling, 2600 m, H Smith 10398 (BM, S); Kwang-yun Hsien
& vicinity, 1800 m, outside cottage, tree, F. T. Wang 22602
(A); W of Wen-chun Hsien 2450 m, streamside, F. T. Wang 21114
(A); E(O-)mei-hsien, Mt. Emei, W. K. Hu 8176
(A), 8243 (A, US), 8786 (A); Yu-shih Liu 1136D (A,
NA); Yachow 1600 ft, T.C. Peng 502 (A); 2000 m, F. T. Wang
23656 (A, P); mt. slope, 2600 m, T. T. Yu 667 (A); mt slope
among woods, 2500 m, T. T. Yu 869 (A); T. C. Lee 3237 (A),
3347 (US); W.P. Fang 18420 (A); morph from Mt. Emei
similar to T. baccata var. adpressa: W. K. Hu 8177
(A), 8497 (A), 8542 (BH); T.C. Lee 4445 (A), 4500
(A), Chiao & Fan 464 (A, K, P, US), W. P. Fang 15128
(A), 15940 (A), 16082 (A), 18310 (A), L.Y. Tai
1117 (A); S. Wushan, ravine, Wilson 624 (A, K); W of Wen-chuan
Hsien, 2800 m, ridge of thicket, F. T. Wang 20993 (A). Hubei
(Hupeh), Western: Shennongjia For. Dist., 31º30’N, 110º30’E, NE of
Guanmenshan along the S side of the Shicao river, 1150 m, Sino-Amer.
1980 Exped. 777 (A); 31º30’N, 110º30’E, S of Jiuhuping Forest
Farm along Jizigou canyon bottom, 1900 m, Sino-Amer. 1980 Exped. 1540
(A); vicinity of Shibapan, 1850 m, Sino-Amer. 1980 Exped. 1824
(A); Shenlungkai, Cho 76099 (A); Henry 6913 (BM, K, US). Yunnan:
Ta-hon-shan near Ta-koo, NE of Likiang Snow Range, by stream in forest, K.
M. Feng 630 (A). Guizhou (Kweichow): Cavalerie &
Foriupat 2604 (P); Yinjiang Xian, vicinity of Xiapingsho on the W
side of the Fanjing Shan Mt. Range, 1100-1400 m, Sino Amer. Guizhou
Bot. Exped. 1854 (GH). Anhui (Anhwei): Southern, Clas Hara
Shan, R-C Ching 2622 (A). Guangxi (Kwangsi): 2110 m, Steward
& Cheo 947 (BM, P). Zhejiang
(Chekiang): Siachu, open cultivated hilltop, 2600 ft, rare, tree 50 ft,
1.2 ft dbh, R- C. Ching 1676 (P).
|
|
 
 
China—E Sichuan: Wushanhsien, 2000–3000 m,
Henry 7097 ((A), Proposed isolectotype for T. baccata
var. sinensis. In top left photo note slight
radial arrangement of leaves near branch apex, compare with
T. umbraculifera var. microcarpa. In top right
photo grayish scale scars appear at base of reddish orange branchlets; compare with T. wallichiana. Illustration
attached to specimen scanned separately and shown in lower photo;
the abaxial leaf margin is indicated to have 7 rows of smooth marginal
cells followed by 5 rows of papillose cells and 12 rows of stomata;
papillae on abaxial midrib cells are shown to be alternate;
epidermal cells in x-section are shown to be elliptical and
indicated to be 20
µm tall and 50 µm wide. Image to the
right, Fig. 2 reproduced from Kwei & Hu (1974), showing abaxial leaf
surface, midrib and stomata bands x65. |



Hubei
vicinity of Shibapan, 1850 m, Sino-Amer. 1980 Exped. 1824
(A). Illustration on packet in top photo scanned separately and
shown in lower photo. Center photo shows close-up of pointed
seed and minute, broad-deltoid, yellowish green scales, and also grayish
scale scars at base of branchlets. The abaxial leaf surface
is indicated to have 8 rows of smooth
marginal cells followed by no papillose cells and 12 rows of
stomata; papillae on abaxial midrib cells are indicated to be
medial; epidermal cells in x-section are shown to be elliptical, 20
µm tall and 37 µm wide. |
 

Hubei:
NE of Guanmenshan, Sino-Amer. 1980 Exped.
777 (A). Illustration on packet in top photo scanned separately
and shown in lower photo. Note minute, broadly deltoid,
yellowish green scales at base of
yellowish green branchlets in right photo. Illustration
indicates
abaxial leaf surface has 7 rows of smooth marginal cells
followed by 4 rows of papillose cells and 13 rows of stomata;
papillae on abaxial midrib cells are shown to be alternate;
epidermal cells in x-section are shown to be elliptical, and
indicated to be 20
µm tall and 25 µm wide.
|
 

Yunnan: NE of Likiang Snow Range, Feng 630 (A).
Photo attached to top photo scanned separately and shown in lower
photo. Note absence of scales at base of branchlets.
Photo is of abaxial midrib taken at 250x. Papillae somewhat
alternate in a single file on narrow epidermal cells.
Sichuan: Isotype, leaf x-section 250x,
showing reddish elliptical epidermal cells.
|
 
 

China—Sichuan: E(O-)mei-hsien, Mt. Emei,
Yu 869 (A). Photo attached to
specimen shows abaxial midrib cells taken at 400x; note marginal
papillae and poorly differentiated stomata band from the midrib. Photo on upper right shows small grayish scales at base of
some branchlets. Illustration of leaf x-section shows rectangular
epidermal cells, and abaxial 4 marginal cells without papillae and
notes there are 12 stomata rows. Stomata band shown in bottom
photo.

Zhejiang:
LiShui. LS001
Identification features applied:
Leaves relatively thick and short, recurved from base to apex, the
younger ones more obtuse to apex than older leaves, not clasping the
branchlet at base and not spreading in the same plane as the
branchlet. Taxus mairei, in contrast, has more sharply
pointed leaves, the younger ones acuminate to acute at apex.
|

China—Sichuan:
E(O-)mei-hsien, Mt. Emei,
Fang 18420 (A). Photo attached to specimen shows part
of stomata band and midrib, the midrib cells with marginal papillae.
Illustration of leaf x-section shows rectangular epidermal cells
indicated to be 12 µm tall and 25µm wide,
and abaxial margin of 4 cells across without papillae and 8
papillose and 12–13 stomata rows. Palisade parenchyma cells are noted
to be 100 µm long, each cell ~45 µm wide.
|
  China—Sichuan: Kwang-yun Hsien,
1800 m; Wang 22602 (A). Right photo shows slightly larger
cuspidate reddish brown
scales at base of branchlets as often seen in T. wallichiana.
Leaves often appear widest above the mid region while also slightly
longer than usually seen for T. chinensis. The
phyllotaxy and leaf shape suggest T. chinensis. Illustration attached to specimen indicates leaf
epidermal cells as seen in x-section are 25–30
µm- wide and 12–15
µm tall, midrib papillae are marginal and
there are 12 stomata rows.
|
 


Guizhou: W
side of the Fanjing Shan Mt. Range, 1100-1400 m, Sino Amer. Guizhou
Bot. Exped. 1854 (GH). Photos attached to specimen scanned
separately as shown at bottom, shows abaxial midrib, stomata band in
part, and adjacent marginal cells. Center photo shows minute
scales at base of branchlets and close-up of seeds. The
illustration attached to specimen indicates a leaf has 14 marginal
cells, medial papillae on accessory cells and marginal papillae on
midrib. |
|
The name T. chinensis was once used for any yew occurring
naturally in China (Rehder 1940), and also Taiwan, Philippines, and
Indonesia (Wilson 1926)—until the earlier legitimate names—that had
been misclassified in Cephalotaxus and Tsuga—were
applied; the code (Art. 11.4, 11.5) requires that the earliest epithet
be adopted regardless of the genus it was erroneously assigned
to—unless conserved. Rehder (1936), for example, discovered one—Tsuga
mairei Lemée & Lév., but continued to use his name, T.
chinensis, whereas Parlatore (1868) and Pilger (1903, 1916) had
reported several earlier names (Cephalotaxus sumatrana, Cephalotaxus
celebica) whose epithets were eventually adopted, T. celebica
(Li 1963), T. sumatrana (de Laubenfels 1978); however, the
correct name for a single subtropical species as applied by these
authors would have to be T. wallichiana.
Not all yewologists accept just one species of Taxus in
southern China (Table 1); Florin (1948a), for example, felt there
were at least two: T. chinensis, which he treated as a variety of
T. wallichiana with distribution primarily in Sichuan, and
another that he considered a new species, T. speciosa, which was
not entirely new since it had been earlier described—as already
indicated, and Florin himself mentioned the names in synonymy (Cheng
& Fu 1978). Florin’s two species were distinguished by the
presence or absence of papillae on the abaxial leaf midrib, and var. chinensis
was further distinguished from var. wallichiana (in the
Himalayas) by the relatively shorter (oblong v. linear) leaves. He also
indicated that T. baccata var. sinensis was synonymous
with var. chinensis according to communications he had with Orr
at Edinburgh who had sent him leaves of Henry’s collections (Henry
7155), which Henry himself had named var. sinensis. Although
I have not studied the Edinburgh specimens of Henry’s collections, the
type was selected based on material at E sent to Florin who cited Henry
7097, 7155.
Chinese botanists have since recognized up to four species and one
variety of Taxus in China (Cheng & Fu 1978); however, they
have misapplied and illegitimately combined previously known names.
These included T. chinensis var. mairei (Lemée &
Lév.)
W. C. Cheng & L. K. Fu (illegit.), T. wallichiana (misapplied
to T. contorta), and T. yunnanensis (superfluous for T.
wallichiana). Hu (1964) followed Florin’s treatment (1948a) except
she maintained T. chinensis as a species. Cheng & Fu (1978),
however, reduced it again to a variety, but not according to ICBN as
just indicated. Taxus chinensis and T. mairei were
considered to differ only as varieties because the distinguishing
feature—presence or absence of papillae on the leaf midrib
(undersurface)—could not always be clearly decided due to the
occurrence of intermediates (Kwei & Hu 1974; Cheng
& Fu 1978),
and also because the type for Cephalotaxus celebica Warb.
had not been studied (Hu 1964; Cheng & Fu 1978). Hu (1964) concluded
that the type for T. speciosa did not significantly differ from
that of Taxus (Tsuga) mairei, whereas the type for Cephalotaxus
celebica might differ because the only specimen she saw from the
Celebes Islands (Neth. Ind. For. Serv. bb:19577, GH) had leaves
with a papillose ventral (abaxial) midrib, and in her opinion this was
distinct from the types of T. mairei and T. speciosa.
I have studied the same specimens that Hu studied at Harvard
(annotated by Hu, July 1955), and largely concur with her
identifications of T. chinensis (Hu 1964). Additionally, I have
studied other specimens from Sulawesi, namely the type for Podocarpus
(Taxus) celebicus Hemsley (K), Teyrmann 14190 (U),
and a photocopy of a holotype fragment for Cephalotaxus (Taxus)
celebica Warb. (S); these have similarly shaped leaves with a
smooth midrib and broad marginal area of partially papillose cells on
the abaxial surface; the “Neth. Ind For.” specimen (GH), in
contrast, differs in having a papillose midrib as noted by Hu (1964).
Based on Florin’s (1948a) account, and the similarity in leaf shape of
the two type specimens—in which I have recognized T. sumatrana celebica
by its long acuminate leaves (tapering from mid region; e.g., H.
Smith 10401 [BM], Plate 6 in Florin, 1948a), I see no reason to
disagree with Florin—that the Warburg type for T. celebica
lacks papillae along the abaxial midrib; thus, while Cheng & Fu
(1978) could have adopted this name in their revised treatment of
Taxaceae in the Flora of China, the correct name under their
species concept is T. sumatrana; the basionym was mentioned by
Pilger (1903, Cephalotaxus sumatrana) who was cited by Cheng & Fu
(1978).
Currently, I distinguish T. chinensis by the pale yellowish
green or “yellowish ochre” to “bronze” (“Prismacolor” chart)
color on older branchlets (“dun colored”; Orr 1937), and by the
relatively short (oblong), thick leaves, usually with conspicuous midrib
papillae along epidermal cell walls. In making this distinction, I have
independently reached the same conclusion as that by Pilger (1903,
1916), Orr (1937), Florin (1948a), and Hu (1964) for recognizing this
taxon by its leaf and bud-scale characteristics, and also that by Orr
(1937) for its branch color. Although branchlets of T. wallichiana
vary in color from reddish orange to purplish, they lack this yellowish
pigmentation, or are not yellowish green.
Taxus sumatrana and T. mairei also have similar
bud-scales, and while they differ from T. chinensis in lacking
papillae on the abaxial midrib and marginal cells—with rare
exceptions, they show other differences. Their branchlets arise
isodichotomously, often in a zigzag arrangement, compared to irregular,
or anisodichotomous branching without a distinct pattern in T.
chinensis, and their abaxial leaf surface has a truncated midrib,
compared to a rounded (keel) midrib in T. chinensis.
Most specimens of T. chinensis can be distinguished from
the Sumatrana Group in China by these features under a standard
dissecting scope, but occasional specimens require microscopic
examination of leaf sections to identify the distribution of papillae on
the abaxial midrib and marginal regions as a confirmatory character
trait. Confusion is mostly likely to occur with Taxus sp. (T.
kingstonii Spjut ined.).
I also see T. chinensis as part of a species complex (Subgroup
Chinensis, Spjut 2000) that is geographically and morphologically
central with regard to the T. cuspidata alliance in northeastern
temperate Asia, a Sumatrana Group centering in southeastern
China, and a Wallichiana Subgroup in eastern Himalayas—as
discussed to some extent earlier under T. wallichiana.
Leaves of T. chinensis frequently have a wider marginal
region of cells without papillae on the abaxial surface—from 4–12
cells wide, occasionally angular epidermal cells as seen in x-sect.
(from Guangxi, Sichuan, Yunnan, and Hubei), occasionally are linear in
shape (W Hubei), and rarely are less papillose on the abaxial midrib
cells (e.g., Hiep & Chan 405 from Vietnam, P). The marginal
border of cells are usually thick-walled and trapezoidal in shape as
seen in the T. cuspidata alliance, whereas specimens with longer
leaves and/or angular shaped epidermal cells in transverse section are
clearly related to T. wallichiana, or those with less papillae on
midrib cells indicate closer ties to T. sumatrana.
The evolutionary relationship of T. chinensis to species of
the T. cuspidata alliance is evident in the variation of the
character traits they share. They
are differentiated from the Wallichiana Group by elliptically
shaped epidermal cells in x-section (Spjut 1998a, 2000a,c).
Among those related to T. cuspidata are plants with a
shrubby habit that have ascending to erect imbricate leaves—typified
by T. caespitosa Nakai (e.g., Makino 43792 from Japan, S,
topotype). Their similarity to T. chinensis is evident on the
abaxial surface of leaves having a rounded keeled midrib (x-section) and
a smooth marginal region of 8–12 trapezoidal cells, and by seeds being
more sharply tapered to apex in contrast to those of T. sumatrana.
Taxus caespitosa varies more in development of papillae on
the undersurface of leaves, especially on the midrib, whereas T.
chinensis more often has a narrower marginal border of smooth cells,
4 cells wide. This
variation suggests a gradual differentiation of T. chinensis from
T. wallichiana and T. caespitosa, which also has further
differentiated itself by development of smaller seeds (shaped like a
‘Hershey Kiss’), by the conspicuous bubble like papillae along cell
walls in T. chinensis appearing reduced to low concrescent ridges
in T. caespitosa, and by loss of stomata.
Among the T. cuspidata alliance are plants with leaves
arranged in nearly two-ranks (e.g., Wilson 10519, from South
Korea, A BM GH US). They
appear more closely allied to T. sumatrana than to T.
chinensis. Their habit is typically a tree (T. biternata Spjut) in
mixed conifer hardwood forests in northeastern temperate Asia (Kolesnikov
1935) with much divided branchlets as in T. mairei, which differs
by a more isodichotomous arrangement, often in a distinct zigzag pattern
(e.g., Fig. 11 in Liu 1960; Tsang 20694 from Guandong, China, A,
US). They also share with T.
sumatrana the flattened to slightly rounded midrib as seen in
x-section, a wider marginal region of epidermal cells without
papillae—from 8–24 cells across, and a larger subglobose seed that
differs by being angled near apex.
The differences between T. sumatrana and this T.
cuspidata complex also appear clinal from southeastern China to
southeastern Russia and Japan (Spjut 2000c, unpubl.). This may relate to
paleoclimatic gradients in that region during the Pliocene and
Pleistocene. Thus, I
recognize two lineages for the Cuspidata Alliance in eastern
Asia.
A specimen of T. chinensis cited from Vietnam and others more
frequently collected on Mt. Emei (Sichuan) were recognized in my
annotations (A, GH) as being distinct for their “complanate” leaf
arrangement. This variant
generally has a relatively wider abaxial leaf margin—from 7–12 cells
across without papillae, and bears strong resemblance to T. baccata
var. adpressa Hort. ex Carrière, which I consider a variety of
T. canadensis (Spjut 2000b). While
it may have been derived from the T. chinensis/T. caespitosa complex,
the similarity to European plants is remarkable in branching, leaf
shape, and leaf anatomy (e.g., Summerhayes 2581 from near Kent,
England, K), including a fossil from an Upper Miocene deposit (Kvaček 1986, “Taxus sp. 2”). The
European plants seem to differ only by fewer stomata rows/band, and
appear to intergrade with the European form of T. canadensis.
Variety adpressa has also been thought to occur in Japan (Endlicher
1847), but it probably originated as a chance seedling of T. baccata
in a nursery at Chester, England sometime around 1826 (Pilger 1916;
Wilson 1916). Nevertheless, plants near the border of Russia, China and
North Korea that have relatively short obtuse leaves and distinct
divaricate branching may prove to be equally related (e.g., Palezevski
88, BM, K) to both European and Asian morphs. Within this region, a
distinct variety of yew has been recognized by its habit as a low
flat-topped, rounded bush that spreads by layering, T. cuspidata
var. microcarpa (Trautv.) Kolesnikov (1935). Its phyllotaxy, as
might be measured by the frequency at which leaves develop along a
branchlet, appears intermediate between T caespitosa and T.
cuspidata. One may
speculate that the Manchurian shrub yew may have been derived from a
former T. canadensis and T. sumatrana species alliance
that occurred in eastern Asia, or perhaps from a T. canadensis
clade that once stretched across Eurasia and eastern North America.
The Chinensis complex also extends into Indonesia and the
Philippines where consistent morphological differences merit taxonomic
distinction from T. chinensis.
I recognized one species (T. phytonii Spjut ined., type
from Philippines, de Laubenfels P668, A) by the relatively
flaccid (drooping) branches and leaves (Fig. 1 in de Laubenfels 1988),
and by the leaves in herbarium specimens showing a sharp contrast in
color between upper and lower surfaces—dark reddish (resinous) to
blackish green above, and pale yellowish green to yellowish orange below
on stomata bands, demarcated by narrow reddish midrib and marginal
zones. The leaves along the abaxial marginal zone lack papillae across
(2-) 4–6 (-8) epidermal cells that have relatively thin-walls and are
slightly inflated. The
number of stomata rows/band has a narrower range—from (10-) 11–14
(-16) rows. In leaf
x-sections, the epidermal cells appear slightly inflated on the adaxial
surface—with a distorted angular shape. Several varieties were also recognized by differences in
shape and size of axillary buds or bud-cones, and by differences in leaf
color and arrangement. Affinities to T. wallichiana are seen in
the linear-falcate acuminate leaves, nearly isodiametric epidermal cells
on the adaxial leaf surface, the relatively narrow region of smooth
marginal cells on the abaxial leaf surface, and in the slightly
cuspidate bud-scales that persist at base of branchlets. Affinities to T.
chinensis are seen in the yellowish tint on branchlets, loss of
papillae on midrib cells—especially on the lower half of the abaxial
leaf surface, and in cross sectional shapes of leaves. The majority of
the specimens were collected in the Philippines, but rare specimens from
Sulawesi (Neth. Ind. For. Serv. 20887, K), Taiwan (Wilson
11154, A), Yunnan (e.g., Tsai 59874, A) and northeastern
India (Ludlow & Sherriff 3719, BM) are remarkably similar.
Other specimens show various combinations of the taxonomic features
that I employ to differentiate T. wallichiana, T. chinensis
and T. sumatrana. They—occurring
relatively infrequent among the collections—generally have turgid
leaves with elliptically shaped epidermal cells in transverse-section,
but vary in other aspects. Included
are specimens that could easily be referred to T. wallichiana (e.g., Oliver from Ruby Mines in Myanmar at different elevations, three
sheets, K), and others that appear more distinct but may be hybrids with
T. sumatrana (e.g., Williams 1054 from Nepal, BM!), or T.
chinensis as further discussed here.
An example of one species under study resembles T. wallichiana
by shape, arrangement and color of leaves, and T. chinensis in
branch color and lack of persistent bud-scales (e.g., Poilane 4150 and
Schmid s.n. from South Vietnam, T. aff. chinensis in adnot.,
A, P; T. rehderiana Spjut ined. as shown elsewhere on this
website). Another mentioned earlier under T. wallichiana from Mt.
Emei has these character features reversed
(T. scutata Spjut ined., e.g., Tsai 58464 from
Yunnan, A, P; Sino-Amer. 1980 Exped. 585 from W Hubei, GH;
Hu 8619 from W Sichuan, A).
A third taxon under study is identified by the leaves showing
less contrast in color between upper and lower surfaces with further
differences in leaves being rigid (e.g., Neth. Ind For. bb:19577
from Sulawesi, GH; C-j. Chang s.n., from Hualien, Taiwan, wba
[private herb.]) or flaccid (e.g.,
de Laubenfels 669, 670 A and C-j Chang s.n. from Taiwan,
wba).
Several specimens from Sichuan and Zhejiang are included under T.
chinensis despite their deviation in a number of features (Chiao
& Fan 464, A, P, US; Ching 1676, A, P, US; Chens s.n.
& locality, P). They
show strong similarity to T mairei in branching, leaf
arrangement, leaf shape (in length and in x-section), and in the
strongly compressed epidermal cells; however, I gave more weight to the
conspicuous papillae positioned marginally on the abaxial midrib
epidermal cells, and to a relatively narrow marginal zone of cells
without papillae–from 7–10 cells across. I considered these features
taxonomically significant based on their apparent ancestral
relationships. This morph
is similar to the extinct T. engelhardtii Kvaček (1986) that was
described from a late Oligocene deposit in Europe except that T.
engelhardtii has more widely spaced stomata in fewer rows (7–10
rows fide Kvaček),
although this type of stomata band appears evident in an extant species
in Sichuan and Yunnan (T. florinii Spjut ined).
CONCLUSION
Taxus chinensis, T. contorta, T. wallichiana and related
species are believed to have evolved in eco-geographically distinct
regions that had remained relatively stable over a long period of time
during the Tertiary,
most likely during the Paleocene and Eocene.
The increasing aridity and cooler climate during the Neogene (Axelrod
1975) may have led to fragmentation of former clinal patterns of
branching and leaf characteristics of Taxus as seen today between
temperate and subtropical species complexes in Asia. Taxus chinensis
occupies a central position to these various, poorly understood
complexes.
It should be noted that species of vascular plants have been
recognized in Tertiary deposits to occur more widely across oceanic
barriers that now exist (Manchester
1999). Many examples are comparable to the distribution of extant
species of Taxus (e.g., T. canadensis; Spjut submitted).
This may help explain why Pilger’s (1903) subspecies of Taxus,
which have generally been accepted as species, appear to defy
morphological classification, and have not received support from
molecular data, except for North American species. Their extant
distribution patterns are also comparable to bryophytes in which their
longevity, survival by vegetative reproduction, and variable leaf
anatomy, indicate a slow rate of evolution (Schofield & Crum 1972).
Since there is a lack of evidence for reproductive isolating
characters in Taxus—other than eco-geographical isolation,
hybridization might be expected where species populations are sympatric.
Taxus chinensis possibly evolved from a hybrid complex between the
Cuspidata alliance and the Wallichiana Group (see Spjut
2007a). Also, the oscillating climate changes during the
Pleistocene may have led to frequent genetic exchange between formerly
isolated populations of Taxus that resulted in the various leaf
anatomical combinations of features that are evident in Asian Taxus.
Leaf anatomical characters are also show the greatest variation
in Asia (Spjut 2007a).
Assuming that both Tertiary intermediates and Pleistocene hybrids
survive—with little change in their morphology, differentiating
between such entities may be more of academic interest than of taxonomic
value. Arriving at a reasonable taxonomic solution requires sifting out
the most common or distinct morphological pattern characteristics. Molecular analyses may eventually demonstrate the complexity
of the problem, but for traditional species classification purposes,
morphological characteristics of taxonomic value are required in order
to draw comparisons to type specimens. And while molecular data might
aid in elucidating phylogenetic relationships, I believe such data must
be guided by morphological criteria, not by geographical criteria
alone—because it is becoming increasingly evident that species of Taxus
in Asia have evolved more in response to ecological differences than to
geographical isolation.
Finally, the taxonomic complexity in the genus suggests that species
and varieties may need to be distinguished by a suite of morphological
features for which no single character can be relied upon entirely.
Rare variants then have to be evaluated on the basis of
uniqueness and whether there is evidence for an evolutionary basis as
seen from a study over the entire range of the genus.
ACKNOWLEDGMENTS
I thank Drs. Roy Vickery and Charlie Jarvis for making specimens and
rare books available at BM, providing photocopies of literature, and
allowing specimens to be photographed; Dr. Ph. Morat at P for making
available their specimens, and allowing them to be photographed; Dr.
Arne Anderberg at S for his communications, hardcopies from their online
database of Taxus specimens, and loan to US; Dr. Zi-yu Cao at PE
for photocopies and leaves of “isotype” of T. yunnanensis,
and correspondence; Dr. Umberto Mossetti at BOLO for providing leaves of
their type of T. orientalis, and its illustration by Bertoloni;
Dr. Patricia Holmgren for providing photocopies of their types of T.
wallichiana, and Dr. Alfred Schuyler for allowing me to study of
specimens at PH; similarly, I am grateful for the assistance from Peter
Edwards at K during my visit; Peter Mazzeo (retired) at NA for obtaining
loans from A, BH, BM, GH, K, M, and U, George Russell and Katherine
Rankin at US for obtaining and maintaining loans from S, and for study
of their collections; and curators at E who have provided a loan of
original material of T. lindleyana.
Thanks also to Dr. Dan Nicolson for helpful discussions on
nomenclatural matters and review comments of the manuscript; to Dr. John
Thieret for his twice generous editorial review and comments, to the
National Agricultural Library for assistance in obtaining rare journals;
the Library of Congress in Washington, D.C.; to the USDA Pacific
Northwest Research Station and USDA Forest Service in that region for
providing fresh material of T. brevifolia and for field
assistance; Kenneth Cochran at the Secrest Arboretum for field
assistance and providing specimens; and to John Wiersema at the USDA
Systematic Botany Laboratory in Beltsville for discussions on
nomenclature in the early stages of this paper.
LITERATURE CITED
Anonymous. 1913. Kew Bull.
1913: 255-263.
Anonymous. 1994. Gymnosperms of Sichuan.
Department of Biology, Sichuan United University.
Appendino, G. 1995. The phytochemistry of the yew
tree. Nat. Prod. Rep. 12: 349-360.
Arnott, G. A. 1838. Ann. Nat. Hist. 1:
130.
Axelrod, D. I. 1975. Evolution and
biogeography of Madrean-Tethyan sclerophyll vegetation. Ann. Missouri
Bot. Gard. 62: 280–334.
_________.
1976. History of the coniferous forests, California and Nevada. Univ.
Calif. Press, Berkeley, 1–62.
_________.1986. Cenozoic
history of some western American pines. Ann. Missouri Bot. Gard. 73:
565–641.
Bertoloni, A. 1862.
Miscellanea botanica XXIII. Mem. Acad. Sci. sér 2: .228–229, tab.
Bertrand, C. E. 1874.
Anatomie comparée des tiges et des feuilles chez les Gnétacées et les
Conifères. Ann. Sci. Nat. Bot. sér. 5, 20: 6–153.
Bobola, M. S., R. T.
Eckert, A. S. Klein, K. Stapelfe, D. E. Smith and D. Guenette. 1996.
Using nuclear and organelle DNA markers to discriminate among Picea
rubens, Picea mariana, and their hybrids. Can. J. For. Res.
26: 433–443.
Brummitt, R. K. and C. E.
Powell. 1992. Authors of plant names. Royal Botanic Gardens, Kew,
England.
Burkhill, I. H. 1965.
Chapters on the history of botany in India. Botanical Survey of India,
Calcutta.
Champion, H. G. and S. K.
Seth. 1968. A revised survey of the forest types of India. Manager of
Publications Delhi, India Press, Nasik.
Chapman, A. W. 1860. Flora
of the southern United States. Ivison, Phinney & Co., New York.
Cheng, W-c., L-k. Fu. and
C-y. Cheng. 1975. Gymnospermae Sinicae. Acta Phytotax. Sin. 13(4):
56–90 & 66 illus., 17 plates.
__________ and L-k. Fu.
1978. Taxaceae. In: Flora Reipublicae Popularis Sinicae. Tomus 7, Gymnospermae. Agendae Academiae Sinicae. [English
Translation].
Cox, E. H. M. 1945.
Plant-hunting in China. Collins, London.
Croom, E. M., Jr. 1995. Taxus
for taxol and taxoids. In M. S. Suffness, ed. Taxol. Science and
applications. CRC Press. Pp. 37-70.
Deryugina, T. F. and N. D.
Nesterovich. 1981. Peculiarities of the morphological and anatomical
structure of conifer needles of some Taxus L. species. Dokl. Akad.
Nauk Bel. S.S.R. 25(7): 652-655 [Russian with English summary].
Dilcher, D. L. 1969. Podocarps from the Eocene of North America. Science
164: 299-301.
Doede, D. L., E. Carroll,
R. Westfall, R. Miller, R. Kitzmiller, J. H. and K. M. B. Snader.
1993. Geographic variation in allozymes and taxol, and
propagation by root cuttings in Pacific yew. In: International yew
resource conference. Yew (Taxus) conservation biology and
interactions: 10, Berkeley, CA (abstract).
El-Kassaby, Y. A. and A.
D. Yanchuk. 1994. Genetic diversity, differentiation, and inbreeding in
Pacific yew from British Columbia. J. Hered. 85: 112-117.
Endlicher, S. 1847.
Synopsis coniferarum. Scheitlin & Zollikofer, Sangalli.
Farjon, A.
1998. A world
checklist of conifers. The Royal Botanic Gardens, Kew.
Ferguson, D. K. 1978. Some
current research on fossil and recent taxads. Rev. Paleobot. Palynol.
26: 213-226.
Florin, R. 1931.
Untersuchungen zur Stammesgeschichte der Coniferales und Cordaitales.
Kongl. Svenska Vetenskapsakad. Handl. 10: 45-588.
__________. 1948a
Enumeration of gymnosperms collected on Swedish expeditions to western
and northwestern China. Acta Horti. Berg. 14: 343-384.
__________. 1948b. On the
morphology and relationships of the Taxaceae. Bot. Gaz. 110: 31-39.
__________. 1958. On
Jurassic taxads and conifers from north-western Europe and eastern
Greenland. Acta Horti. Berg. 17.
__________. 1963. The
distribution of conifer and taxad genera in time and space. Acta Horti
Berg. 20: 121-312.
Franco, J. A. 1964. Taxus.
Fl. Europaea 1: 39.
Frederiksen, N. O. 1994.
Paleocene flora diversities and turnover events in eastern North America
and their relation to diversity models. Rev. Palaeobot. Palynol. 82:
225-238.
__________. 1995.
Differing Eocene floral histories in southeastern North America and
western Europe: Influence of paleogeography. Hist. Biol. 10: 13-23.
Freitag, H. 1971. Studies
in the natural vegetation of Afghanistan. In P. H. Davis, P C. Harper,
and I. C. Hedge, eds. Plant life of south-west Asia. Bot. Soc.
Edinburgh. Pp. 89-106.
Frimmel, F. von. 1911. Die
untere Kutikula des Taxus- Blattes—ein Lichtreflektor. Oesterr.
Bot. Z. 61: 216-223.
Fu, L-k, N. Li and R. R.
Mill. 1999. Taxaceae. Flora of China 4: 89–96, Missouri Botanical
Garden Press, St. Louis.
Gamble, J. S. 1922. A
manual of Indian timbers. Sampson Low, Marston & Co,,London.
Gaussen, H. 1979. Les
gymnospermes actuelles et fossiles: fasc. 15. Les taxines. Toulouse,
Unversité Paul-Sabatier Faculté des Sciences.
Gordon, G. 1875. The
Pinetum being a synopsis of all the coniferous plants at present known,
with descriptions, history and synonyms. 2nd ed. Henry C. Bohn, London.
Graham, A. 1972. Outline
of the origin and historical recognition of floristic affinities between
Asia and eastern North America. In A. Graham, ed., Floristics and
paleofloristics of Asia and eastern North America, Elsevier Publ. Co.,
Amsterdam. Pp. 1-18.
__________. 1973. History
of the arborescent temperate element in the northern Latin American
Biota. In A. Graham, ed., Vegetation and vegetational history of
northern Latin America. Elsevier Scientific Publ. Co., Amsterdam. Pp.
301-314.
__________. 1993. History
of the vegetation: Cretaceous (Maastrichtian)—Tertiary. Fl. North
America 1: 57–70.
__________.
1999. Late Cretaceous and Cenozoic history of North American vegetation
north of Mexico. Oxford
Univ. Press, New York.
Greuter, W., J. McNeill,
F. R. Barrie, H. M. Burdet, V. Demoulin, T. S. Filgueiras, D. Nicolson,
P. C. Silva, J. E. Skog, P. Trehane, N. J. Turland and D. L. Hawksworth.
2000. International code of botanical nomenclature (Saint Louis Code)
adopted by the Sixteenth International Botanical Congress. St. Louis,
MO, July-August 1999. Koeltz Scientific Books, Königstein, Germany.
Grierson, A. J. C. and D.
G. Long. 1983. Flora of Bhutan. Vol 1. Pt. 1. Royal Botanic Garden,
Edinburgh.
Griffith, W. 1854. Notulae
ad plantas Asiaticas. Part IV. Posthumous papers, Govt. Bengal,
Calcutta.
Handel-Mazzetti, H.
1929. Symbolae Sinicae. Julius Springer, Wien.
Hara, H., W. T. Stearn and
L. H. J. Williams. 1978. An enumeration of the flowering plants of
Nepal. Vol. 1. British Museum, London.
Harris, T. M. 1976. The
Mesozoic gymnosperms. Rev. Paleobot. Palynol. 21: 119-134.
Hickman, J. (ed.). 1993.
The Jepson Manual (Appendix I). University of California Press,
Berkeley.
Hils, M. [Spjut in] 1993.
Taxaceae Gray. Yew family. Fl. North America 2: 423–427.
Holmgren, P. K., N. H.
Holmgren and L. C. Barnett.1990. Index Herbariorum. Part I: The herbaria
of the world. 8th ed. W. Junk, The Hague.
Hu, S-y. 1964. [Notes on]
the Flora of China. Taiwania: 13-62.
Jarvis, C. E., F. R.
Barrie, D. M. Allan and J. L. Reveal. 1993. A list of Linnaean generic
names and their types. Regnum Veg. 127.
Kingston, D. G. I. 1996.
Improving on nature. Taxane J. 2: 20-27.
__________, G.
Samaranayake and C. A. Ivey. 1990. The chemistry of taxol, a clinically
useful anticancer agent. J. Nat. Prod. 55: 1-12.
Kolesnikov, B. P. 1935. On
the shrubby kind of the spiky yew (Taxus cuspidata S. et Z.).
Bull. Far E. Branch
Acad. Sci., USSR 31-47 (In Russian with English summary).
Krüssmann, G. 1985.
Manual of cultivated conifers. Translated by M. E. Epp, H.-D. Warda
& G. S. Daniels, eds. Timber Press, Portland.
Kvaček,
Z. [1986 fide author]. Tertiary taxads of NW Bohemia. 1982 Acta Univ.
Carol.,. Geol., Pokorny 4: 471–491.
Kwei, Y-l. & Hu, S.-y.
1974. [Epidermal feature of leaves of Taxus in relation to taxonomy].
Acta Phytotax. Sin. 12(3): 329-334, plate 67 [Chinese with English
summary].
Laubenfels, D. J., de.
1978. The taxonomy of Philippine Coniferae and Taxaceae. Kalikasan 7:
117–152.
__________. 1988.
Coniferales. Fl. Malesiana 10 (3): 337-453.
Léveillé, H. 1914. Monde
Pl. sér. 2, 16: 88, 20.
Li, H-l. 1963. Woody flora
of Taiwan. Livingston Publ. Co., Narberth, PA.
Li, N. and L-k. Fu. 1997.
Notes on gymnosperms I. Taxonomic treatments of some Chinese conifers.
Novon 7: 261-264.
Liberty Hyde Bailey
Hortorium, Staff. 1976. Hortus Third. Macmillan Publishing Co., New
York.
Linnaeus, C. 1753.
Species plantarum: 1040. Laurentii
Salvii, Stockholm.
Liu, T-s. 1960.
Illustrations of native and introduced ligneous plants of Taiwan. Vol. 1. National Taiwan University.
Loudon, J. C. 1844.
Arboretum et fruticetum Britannicum. Longman,
Brown, Green and Longmans, London.
Manchester, S. R. 1994. Fruits and seeds of
the Middle Eocene Nut Beds Flora, Clarno Formation, Oregon. Palaeontogr.
Amer. 58: 1-205.
__________. 1999.
Biogeographical relationships of North American Tertiary Floras.
Ann. Missouri Bot. Gard. 86: 472-522.
Marshall, H. 1785.
Arbustum Americanum. Joseph Crukshank, Philadelphia.
Meyen, S.V. 1984. Basic
features of gymnosperm systematics and phylogeny as evidenced by the
fossil record. Bot. Rev. 5: 1-111.
Miller, C. N., Jr., 1976.
Early evolution in the Pinaceae. Rev. Paleobot. Palynol. 21: 101-117.
___________. 1977.
Mesozoic conifers. Bot. Rev. 43: 217-280.
___________. 1988. The origin of modern conifer families. In C. B. Beck,
ed. .Origin and evolution of gymnosperms. Columbia University Press, New
York. Pp. 448-486.
Miquel, F. 1859. Flora
Indiae batavae. 2: 1076. C. G. van der Post, Amsterdam.
Nasir, E. and S. I. Ali.
1987. Gymnospermae. Flora of Pakistan. Nos. 178–186, E. Nasir & Y.
J. Nasir, eds. Pakistan Agricultural Research Council, Islamabad.
Nuttall,
T. 1849. North Amer. Sylva 3: 86–89.
Orr, M. Y. 1937. On the
value for diagnostic purposes of certain of the anatomical features of
conifer leaves. Notes Roy. Bot. Gard. Edinburgh 94: 256-265 & Plates
256–258.
Parlatore, P. 1868.
Coniferae. In A. de
Candolle, Prodr. syst. nat. reg. veg. 16 (2): 361-524.
Pilger, R. 1903. Taxaceae-Taxoideae—Taxeae.
Taxus. In Engler, Das Pflanzenreich IV: 110–116.
__________. 1916. Die
Taxales. Mitt. Deutsch. Dendrol. Ges. 25: 1–28.
__________. 1926.
Taxaceae. In Engler, A. and K. Prantl (eds.), Die natürlichen
Pflanzenfamilien, 2nd ed., 13: 199–211.
Prance, G. T. 1982. A
review of the phytogeographic evidences for Pleistocene climate changes
in the neotropics. Ann. Missouri Bot. Gard. 69: 594–624.
Rao, A. R. and M. Malaviya.
1965. On the distribution, structure, and ontogeny of sclereids in Taxus
baccata. Linn. Proc. Natl. Inst. Sci. India 31: 114–122.
Rau, M. A. 1974.
Vegetation and phytogeography of the Himalaya. In M. S. Mani, ed.
Ecology and biogeography of India, W. Junk, Hague. Pp. 247–280.
Rehder, A. 1919. New
species, varieties and combinations from the herbarium and the
collections of the Arnold Arboretum. J. Arnold Arb. 1: 44-60.
__________. 1936. Notes on
the ligneous plants described by H. Léveillé from eastern Asia.
Taxaceae. J. Arnold Arb. 17: 54.
__________. 1940. Manual
of cultivated trees and shrubs. 2nd ed. Macmillan Co., New York.
__________. 1949.
Bibliography of cultivated trees and shrubs. Arnold Arboretum, Jamaica
Plain, MA.
Sapru, B. L.1975.
Vegetational studies in Jhelum Valley. Botanique 6: 151-164.
Sargent, C. S. 1914.
Plantae wilsonianae. Vol. 2. Cambridge University Press.
Schlechtendal, D. F. L.
1838. Vorläufige Nachricht die mexicanishen Coniferen. Linnaea 12: 496.
Schofield, W. B. and H. A.
Crum. 1972. Disjunctions in bryophytes.
Ann. Missouri Bot. Gard. 59: 174-202.
Siebold, P. F., de and J.
G. Zuccarini. 1843.
Plantarum, quas in Japonia collegit Dr, Ph. Fr. de Siebold. Genera Nova.
Notis characteristicis delineatiionibusque illustrata proponunt, I. Abh.
Math.-Phys. Cl. Königl. Bayer. Akad. Wiss. 3.
__________ and __________.
1846. Florae japonicae familiae naturales. Abh. Math.-Phys. Cl. Königl.
Bayer. Akad. Wiss. 4(3): 123-240.
Silba, J. 1984. An
international census of the Coniferae, I. Phytologia Mem. 7: 1-79.
Silber, M. & DeWolf,
G. P., Jr. 1970. Yews in fiction and fact. Arnoldia 30: 139-147.
Spjut, R. W. 1992. A
taxonomic key to the species of Taxus. NCI Workshop on Taxus,
Taxol, and Taxotere, Rockville, MD (Abstract only).
__________. 1993. Reliable
morphological characters for distinguishing species of Taxus
(Abstract). In: International yew resource conference. Yew
(Taxus) conservation biology and interactions: 39-40, Berkeley, CA.
__________. 1998a.
Morphological evolution in the Taxus leaf and its significance to
recognizing ecological species within the genus. Presented at the AIBS
Annual Meeting, American Systematic Plant Taxonomists, Baltimore
Convention Center, MD, Aug. 5, 1998. Abstract published on the Internet,
Botanical Society of America (URL: http://www.botany.org/).
__________. 1998b. Species
of Taxus. Presented at the AIBS Annual Meeting, American
Systematic Plant Taxonomists, Baltimore Convention Center, MD, Aug. 5,
1998. Abstract published on the Internet, Botanical Society of America
(URL: http://www.botany.org/).
__________ 2000. Three papers presented at
the joint meetings of the Botanical Society of America and American
Systematic Plant Taxonomists, Portland, OR, August, abstracts
published online and Amer. J. Bot.
(a) A phytogeographical analysis and classification of leaf
morphological features in Taxus (Taxaceae).
http://www.ou.edu/cas/botany-micro/botany2000/section13/abstracts/27.shtml
(b) The morphological relationships of Taxus canadensis (Taxaceae) in North America
and Eurasia. http://www.ou.edu/cas/botany-micro/botany2000/section13/abstracts/28.shtml
(c) A revised taxonomic key to species and varieties of Taxus (Poster).
http://www.ou.edu/cas/botany-micro/botany2000/section13/abstracts/166.shtml
__________.
2007a. A Phytogeographical analysis of Taxus (Taxaceae) based on
leaf anatomical characters. J. Bot. Res. Inst. Texas 1(1): 291–332.
__________. 2007b.
Taxonomy and nomenclature of Taxus. J. Bot. Res. Inst. Texas.
1(1): 203–289.
__________, Zhou, J-y.
& Chang, C-j. Comparison of taxane content between two different
native yews from the Pacific northwest. Poster/Abstract, 34th Annual
Meeting, American Society of Pharmacognosy, San Diego, CA, Jul 1993.
Srivastava, S. K. 1994.
Evolution of Cretaceous phytogeoprovinces, continents and climates. Rev.
Paleobot. Palynol. 82: 197-224.
Stafleu, F. A. and R. S.
Cowan. 1976-1988. TL-2. Taxonomic literature. 7 Vols. W. Junk, Hague.
Stainton, J. D. A. 1972.
Forests of Nepal. Camelot Press, London.
Vance, N. C. and A. B.
Krupkin. 1993. Using restriction fragment length polymorphisms to assess
genetic variability among yew taxa. In: International yew resource
conference. Yew (Taxus) conservation biology and interactions:
34, Berkeley, CA (abstract).
Voliotis, D. 1986. Historic and environmental
significance of the yew (Taxus baccata L.). Israel J. Bot. 35:
47-52.
Wallich, N. 1826. Tentamen
florae nepalensis illustratae. Fasc. 2. Calcutta and Serampore.
Wang, C-w. 1961. The
forests of China. Maria Moors Cabot Foundation No. 5, Harvard
University, Cambridge, i-ix &1-313.
Wani, M.C., Taylor, H. L.,
Wall, M.E., Coggon, P. and McPhail, A. T. 1971. Plant antitumor agents.
VI. The isolation and structure of taxol, a novel antileukemic and
antitumor agent from Taxus brevifolia. J. Amer. Chem. Soc. 93:
2325-2327.
Warburg. O. 1900. Beiträge
zur Kenntniss der Vegetation des Sǖd
und Ostasiatischen Monsungebietes.
Monsunia I: 194.
Wilkinson, R. C., Hanover,
J. W., Wright J. W. & Flake, R. H. 1971. Genetic variation in the
monoterpene composition of white spruce. For. Sci. 17: 83-90.
Wilson, E. H. 1916. The
conifers and taxads of Japan. Publ. Arnold Arb. 8: 1- 91, Cambridge
Univ. Press, Cambridge.
__________. 1926.
Taxaceae. In: The taxads and conifers of Yunnan, J. Arnold Arb. 7:
39-42.
Wolfe. J. A. 1975. Some
aspects of plant geography of the northern hemisphere during the late
Cretaceous and Tertiary. Ann. Missouri Bot. Gard. 62: 264- 279.
|
|
Table 1 |
Footnote: Peer Reviewed for publication in Sida (2001).
Note: an edited version of this manuscript was resubmitted for peer review in May 2006. Incorporated
into a larger manuscript that has completed peer
review in Dec. 2006 and finally published August 2007.
Presented on the Web: April 2003;
Photos of specimens added May 13-19, and July 22, 2006 Also added: Links to table
on leaf anatomical data
and to an explanation for the
characters May 2006; Taxus fuana reduced to synonomy based on
study of its type, Jan. 2007.
Range of var. wallichiana was extended to SE Tibet based on specimen
received 24 Apr 2007.Additional Footnote (June 2010): First submitted
to Taxon in 1998. The manuscript was not considered. Members of the IAPT who serve on the nomenclatural committee have continued to
support the use the
illegitimate names of Taxus and to justify other names of Taxus
without taxonomic merit. A good example is the MBG and USDA who have
gone to extreme measures to justify use of T. fuana by recognizing
T. contorta being found only in Afghanistan. Earlier Farjon
had tried to justify T. fuana by recognizing as endemic to
the type locality in Tibet. The author of this website does not support
such nonsense and has discontinued his membership in
the IAPT as a result. Also, this website has been attacked by hackers who
have deleted images and references to images, which have had to be
restored on number of occasions from back-up files. |
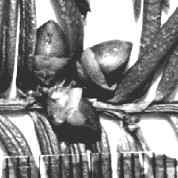

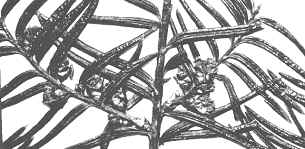



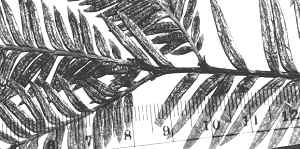




























































 used in a tea in Ladakh, det. by Spjut as T.
contorta, designated by Spjut 2007). Note that both publications by Griffith have to be
applied together for valid publication.
used in a tea in Ladakh, det. by Spjut as T.
contorta, designated by Spjut 2007). Note that both publications by Griffith have to be
applied together for valid publication.



















































 3.
3.

























